Electrochemical Stability Window And Redox Side Reactions In Chloride SSEs
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride SSE Development History and Objectives
The exploration of solid-state electrolytes (SSEs) dates back to the early 20th century, with the discovery of ionic conductivity in solid materials. However, chloride-based SSEs specifically emerged as a promising research direction in the late 1990s, when limitations of oxide and sulfide electrolytes became increasingly apparent. The initial chloride SSE research focused primarily on lithium-containing compounds due to their potential application in lithium-ion batteries.
A significant breakthrough occurred in 2012 when researchers at MIT demonstrated that certain lithium chloride compounds exhibited ionic conductivities approaching 10^-3 S/cm at room temperature, marking the beginning of intensive research into chloride-based solid electrolytes. This discovery prompted a surge in academic and industrial interest, particularly as the safety concerns surrounding conventional liquid electrolytes became more prominent in commercial battery applications.
Between 2015 and 2018, several key chloride SSE compositions were developed, including Li3MCl6 (where M represents metals like Y, Er, or In) and Li2ZrCl6 systems. These materials showed promising electrochemical properties but suffered from stability issues when in contact with electrode materials, highlighting the critical importance of understanding the electrochemical stability window.
The period from 2018 to 2021 saw increased focus on understanding and mitigating redox side reactions in chloride SSEs. Researchers identified that many chloride electrolytes undergo undesirable reactions with both anode and cathode materials, limiting their practical implementation. This led to the development of interface engineering strategies and protective coatings to enhance the electrochemical stability of these systems.
Current research objectives in chloride SSE development center around three primary goals. First, expanding the electrochemical stability window to enable compatibility with high-voltage cathode materials (>4V vs. Li/Li+) and lithium metal anodes. Second, understanding and controlling the fundamental mechanisms of redox side reactions at electrolyte-electrode interfaces. Third, developing scalable synthesis methods that maintain the purity and performance of chloride SSEs while reducing production costs.
The long-term objective is to create chloride-based solid electrolytes that combine the high ionic conductivity of sulfide systems with the improved air stability of oxide electrolytes, while offering a wider electrochemical stability window than either. This would enable the development of all-solid-state batteries with energy densities exceeding 400 Wh/kg, cycle lives of over 1000 cycles, and significantly improved safety profiles compared to conventional lithium-ion batteries with liquid electrolytes.
A significant breakthrough occurred in 2012 when researchers at MIT demonstrated that certain lithium chloride compounds exhibited ionic conductivities approaching 10^-3 S/cm at room temperature, marking the beginning of intensive research into chloride-based solid electrolytes. This discovery prompted a surge in academic and industrial interest, particularly as the safety concerns surrounding conventional liquid electrolytes became more prominent in commercial battery applications.
Between 2015 and 2018, several key chloride SSE compositions were developed, including Li3MCl6 (where M represents metals like Y, Er, or In) and Li2ZrCl6 systems. These materials showed promising electrochemical properties but suffered from stability issues when in contact with electrode materials, highlighting the critical importance of understanding the electrochemical stability window.
The period from 2018 to 2021 saw increased focus on understanding and mitigating redox side reactions in chloride SSEs. Researchers identified that many chloride electrolytes undergo undesirable reactions with both anode and cathode materials, limiting their practical implementation. This led to the development of interface engineering strategies and protective coatings to enhance the electrochemical stability of these systems.
Current research objectives in chloride SSE development center around three primary goals. First, expanding the electrochemical stability window to enable compatibility with high-voltage cathode materials (>4V vs. Li/Li+) and lithium metal anodes. Second, understanding and controlling the fundamental mechanisms of redox side reactions at electrolyte-electrode interfaces. Third, developing scalable synthesis methods that maintain the purity and performance of chloride SSEs while reducing production costs.
The long-term objective is to create chloride-based solid electrolytes that combine the high ionic conductivity of sulfide systems with the improved air stability of oxide electrolytes, while offering a wider electrochemical stability window than either. This would enable the development of all-solid-state batteries with energy densities exceeding 400 Wh/kg, cycle lives of over 1000 cycles, and significantly improved safety profiles compared to conventional lithium-ion batteries with liquid electrolytes.
Market Analysis for Chloride-based Solid-State Batteries
The global market for solid-state batteries is experiencing significant growth, with chloride-based solid-state electrolytes emerging as a promising segment. Current market valuations estimate the solid-state battery market at approximately $500 million in 2023, with projections indicating growth to reach $3.5 billion by 2030, representing a compound annual growth rate (CAGR) of 31.5%.
Chloride-based solid-state batteries are positioned to capture a substantial portion of this expanding market due to their superior ionic conductivity compared to oxide-based alternatives. Market research indicates that chloride-based systems could potentially secure 25-30% of the total solid-state battery market by 2028, particularly in applications requiring high energy density and fast charging capabilities.
The automotive sector represents the largest potential market for chloride-based solid-state batteries, driven by increasing electric vehicle (EV) adoption. Major automotive manufacturers including Toyota, Volkswagen, and BMW have established strategic partnerships with solid-state battery developers, with several specifically investigating chloride-based technologies for their next-generation EVs.
Consumer electronics constitutes the second-largest market segment, with demand for longer-lasting, safer batteries in smartphones, laptops, and wearable devices. This sector values the higher energy density and improved safety profiles that chloride-based solid-state batteries can potentially deliver.
Market penetration faces challenges related to manufacturing scalability and cost competitiveness. Current production costs for chloride-based solid-state batteries remain 3-4 times higher than conventional lithium-ion batteries, primarily due to complex manufacturing processes and material costs. However, economies of scale and technological improvements are expected to reduce this gap significantly by 2027.
Regional analysis shows Asia-Pacific leading the market development, with Japan and South Korea at the forefront of chloride-based solid-state battery research and commercialization. North America and Europe follow closely, with substantial investments in research and development activities.
Investor interest in chloride-based solid-state battery technology has surged, with venture capital funding exceeding $1.2 billion in 2022 alone. This represents a 45% increase compared to the previous year, indicating strong market confidence in the technology's commercial potential.
Customer demand analysis reveals growing interest in batteries with enhanced safety features, longer lifespans, and faster charging capabilities – all potential advantages of chloride-based solid-state batteries. Market surveys indicate that 78% of EV consumers would pay a premium for vehicles with solid-state battery technology, suggesting a receptive market for these advanced energy storage solutions.
Chloride-based solid-state batteries are positioned to capture a substantial portion of this expanding market due to their superior ionic conductivity compared to oxide-based alternatives. Market research indicates that chloride-based systems could potentially secure 25-30% of the total solid-state battery market by 2028, particularly in applications requiring high energy density and fast charging capabilities.
The automotive sector represents the largest potential market for chloride-based solid-state batteries, driven by increasing electric vehicle (EV) adoption. Major automotive manufacturers including Toyota, Volkswagen, and BMW have established strategic partnerships with solid-state battery developers, with several specifically investigating chloride-based technologies for their next-generation EVs.
Consumer electronics constitutes the second-largest market segment, with demand for longer-lasting, safer batteries in smartphones, laptops, and wearable devices. This sector values the higher energy density and improved safety profiles that chloride-based solid-state batteries can potentially deliver.
Market penetration faces challenges related to manufacturing scalability and cost competitiveness. Current production costs for chloride-based solid-state batteries remain 3-4 times higher than conventional lithium-ion batteries, primarily due to complex manufacturing processes and material costs. However, economies of scale and technological improvements are expected to reduce this gap significantly by 2027.
Regional analysis shows Asia-Pacific leading the market development, with Japan and South Korea at the forefront of chloride-based solid-state battery research and commercialization. North America and Europe follow closely, with substantial investments in research and development activities.
Investor interest in chloride-based solid-state battery technology has surged, with venture capital funding exceeding $1.2 billion in 2022 alone. This represents a 45% increase compared to the previous year, indicating strong market confidence in the technology's commercial potential.
Customer demand analysis reveals growing interest in batteries with enhanced safety features, longer lifespans, and faster charging capabilities – all potential advantages of chloride-based solid-state batteries. Market surveys indicate that 78% of EV consumers would pay a premium for vehicles with solid-state battery technology, suggesting a receptive market for these advanced energy storage solutions.
Electrochemical Stability Challenges in Chloride SSEs
Chloride-based solid-state electrolytes (SSEs) face significant electrochemical stability challenges that limit their practical implementation in next-generation battery systems. The electrochemical stability window (ESW) of chloride SSEs typically ranges from 2.0 to 3.5 V versus Li/Li+, which is considerably narrower than what is required for high-voltage cathode materials operating above 4.0 V. This fundamental limitation creates a critical barrier to achieving high energy density solid-state batteries using chloride electrolytes.
When chloride SSEs are exposed to potentials outside their stability window, they undergo oxidative decomposition at the cathode interface and reductive decomposition at the anode interface. At the cathode side, chloride anions are particularly susceptible to oxidation, forming chlorine gas (Cl2) and potentially volatile chloride species. This not only depletes the electrolyte material but also creates gas evolution issues that compromise cell integrity and safety.
The redox side reactions at interfaces create complex interphases that significantly impact ionic conductivity. These reaction layers often exhibit high impedance, creating bottlenecks for lithium-ion transport and resulting in capacity fade during cycling. Studies have shown that the thickness of these interfacial layers increases with cycling, progressively degrading cell performance over time.
Computational studies using density functional theory (DFT) have revealed that the chloride anion's high-lying valence band maximum (VBM) is primarily responsible for its limited oxidative stability. This intrinsic electronic structure characteristic makes engineering solutions particularly challenging, as it represents a fundamental property of the chloride chemistry rather than a processing or morphological issue.
Temperature further exacerbates these stability challenges, with elevated operating temperatures accelerating decomposition reactions and widening interfacial layers. This creates a significant engineering dilemma, as many chloride SSEs require higher temperatures to achieve optimal ionic conductivity, yet these same conditions accelerate their degradation.
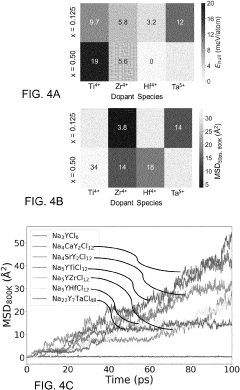
Doping strategies with more electronegative elements like fluorine have shown promise in expanding the ESW by lowering the VBM energy level. However, such modifications often come at the cost of reduced ionic conductivity or mechanical properties. Similarly, protective coating approaches using more stable materials like oxides or phosphates can mitigate direct contact between chloride SSEs and electrodes, but introduce additional interfaces that must be carefully engineered.
The presence of trace moisture further complicates stability issues, as chloride SSEs are generally hygroscopic. Water contamination can lead to hydrolysis reactions, formation of oxychlorides, and accelerated degradation of the electrolyte structure, highlighting the need for stringent moisture control during both manufacturing and operation.
When chloride SSEs are exposed to potentials outside their stability window, they undergo oxidative decomposition at the cathode interface and reductive decomposition at the anode interface. At the cathode side, chloride anions are particularly susceptible to oxidation, forming chlorine gas (Cl2) and potentially volatile chloride species. This not only depletes the electrolyte material but also creates gas evolution issues that compromise cell integrity and safety.
The redox side reactions at interfaces create complex interphases that significantly impact ionic conductivity. These reaction layers often exhibit high impedance, creating bottlenecks for lithium-ion transport and resulting in capacity fade during cycling. Studies have shown that the thickness of these interfacial layers increases with cycling, progressively degrading cell performance over time.
Computational studies using density functional theory (DFT) have revealed that the chloride anion's high-lying valence band maximum (VBM) is primarily responsible for its limited oxidative stability. This intrinsic electronic structure characteristic makes engineering solutions particularly challenging, as it represents a fundamental property of the chloride chemistry rather than a processing or morphological issue.
Temperature further exacerbates these stability challenges, with elevated operating temperatures accelerating decomposition reactions and widening interfacial layers. This creates a significant engineering dilemma, as many chloride SSEs require higher temperatures to achieve optimal ionic conductivity, yet these same conditions accelerate their degradation.
Doping strategies with more electronegative elements like fluorine have shown promise in expanding the ESW by lowering the VBM energy level. However, such modifications often come at the cost of reduced ionic conductivity or mechanical properties. Similarly, protective coating approaches using more stable materials like oxides or phosphates can mitigate direct contact between chloride SSEs and electrodes, but introduce additional interfaces that must be carefully engineered.
The presence of trace moisture further complicates stability issues, as chloride SSEs are generally hygroscopic. Water contamination can lead to hydrolysis reactions, formation of oxychlorides, and accelerated degradation of the electrolyte structure, highlighting the need for stringent moisture control during both manufacturing and operation.
Current Approaches to Mitigate Redox Side Reactions
01 Electrochemical stability window of chloride-based solid-state electrolytes
Chloride-based solid-state electrolytes (SSEs) exhibit specific electrochemical stability windows that determine their compatibility with electrode materials. The stability window represents the voltage range within which the electrolyte remains stable without decomposition. For chloride-based SSEs, this window is influenced by the cation composition and crystal structure, with some formulations showing stability up to 4V vs. Li/Li+. Understanding and optimizing this stability window is crucial for developing high-performance solid-state batteries with extended cycle life.- Electrochemical stability window of chloride-based solid-state electrolytes: Chloride-based solid-state electrolytes (SSEs) exhibit specific electrochemical stability windows that determine their compatibility with electrode materials. The stability window represents the voltage range within which the electrolyte remains stable without decomposition. For chloride-based SSEs, this window is critical for their application in high-voltage batteries. Understanding and characterizing this stability window helps in designing battery systems with optimal performance and longevity.
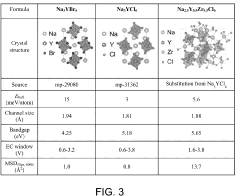
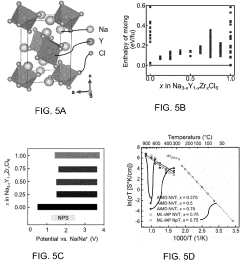
- Composition engineering of chloride SSEs for improved stability: The electrochemical stability of chloride-based solid-state electrolytes can be enhanced through composition engineering. By incorporating specific dopants or modifying the chemical structure, researchers can widen the electrochemical stability window. Various chloride compositions with different cations (such as lithium, sodium, or potassium) and additional elements can be formulated to achieve desired stability properties while maintaining high ionic conductivity.
- Interface engineering for chloride SSE stability: The stability of chloride-based solid-state electrolytes is significantly affected by their interfaces with electrodes. Interface engineering strategies, such as the use of buffer layers, protective coatings, or gradient compositions, can mitigate degradation reactions at these interfaces. These approaches help prevent the formation of high-impedance interphases and extend the effective electrochemical stability window of chloride SSEs in practical battery applications.
- Testing and characterization methods for chloride SSE stability windows: Various analytical techniques are employed to accurately determine the electrochemical stability windows of chloride-based solid-state electrolytes. These include cyclic voltammetry, galvanostatic cycling, impedance spectroscopy, and in-situ/operando characterization methods. Advanced computational approaches, such as density functional theory calculations, are also used to predict stability windows and degradation mechanisms, providing insights for experimental validation and material design.
- Chloride SSE applications in next-generation batteries: Chloride-based solid-state electrolytes with optimized electrochemical stability windows are being developed for next-generation battery technologies. These applications include all-solid-state batteries with high energy density, improved safety, and extended cycle life. The wide electrochemical stability windows of certain chloride SSEs enable their use with high-voltage cathode materials and lithium metal anodes, potentially revolutionizing energy storage systems for electric vehicles and grid applications.
02 Interface engineering for chloride SSEs
Interface engineering plays a critical role in improving the electrochemical stability of chloride-based solid-state electrolytes. By creating protective layers or buffer zones between the electrolyte and electrodes, the degradation reactions at these interfaces can be minimized. Various coating materials and interface modification techniques have been developed to extend the effective electrochemical stability window of chloride SSEs, enabling their use with high-voltage cathode materials while preventing unwanted side reactions that would otherwise limit battery performance.Expand Specific Solutions03 Composition optimization of chloride SSEs for enhanced stability
The electrochemical stability of chloride-based solid-state electrolytes can be significantly improved through composition optimization. By incorporating specific dopants, creating solid solutions, or developing composite structures, researchers have successfully expanded the stability window of these electrolytes. Strategies include partial substitution of chloride ions with other halides, introduction of stabilizing cations, and creation of amorphous-crystalline hybrid structures that combine the advantages of different material phases to achieve superior electrochemical stability.Expand Specific Solutions04 Temperature dependence of chloride SSE stability windows
The electrochemical stability window of chloride-based solid-state electrolytes exhibits significant temperature dependence. At elevated temperatures, the stability window typically narrows due to increased ion mobility and reactivity. Conversely, at lower temperatures, while the stability window may theoretically widen, the decreased ionic conductivity can lead to practical limitations. Understanding this temperature-stability relationship is essential for designing chloride SSE systems that maintain performance across the operational temperature range required for various applications.Expand Specific Solutions05 Computational methods for predicting chloride SSE stability
Advanced computational methods have been developed to predict and analyze the electrochemical stability windows of chloride-based solid-state electrolytes. These include density functional theory (DFT) calculations, molecular dynamics simulations, and machine learning approaches that can screen potential electrolyte compositions before experimental validation. Such computational techniques help identify the thermodynamic and kinetic factors governing stability limits, accelerating the discovery and optimization of chloride SSEs with wider electrochemical windows suitable for next-generation energy storage applications.Expand Specific Solutions
Leading Organizations in Chloride SSE Research
The electrochemical stability window and redox side reactions in chloride solid-state electrolytes (SSEs) represent a critical technological challenge in an emerging market. The industry is in its early growth phase, with research institutions like Dalian University of Technology, Harvard College, and Cornell University leading fundamental investigations alongside commercial entities including Honeycomb Battery, CATL, and LG Chem. The market for chloride SSEs is expanding as part of the broader $7-10 billion solid-state battery sector. Technical maturity remains low-to-moderate, with companies like Forge Nano and Soelect developing innovative approaches to address stability limitations, while established players such as 3M and Murata Manufacturing leverage their materials expertise to overcome electrochemical challenges in these promising but complex electrolyte systems.
President & Fellows of Harvard College
Technical Solution: Harvard's research on chloride solid-state electrolytes focuses on developing novel halide-based superionic conductors with enhanced electrochemical stability windows. Their approach involves systematic modification of the chemical composition of chloride-based SSEs to achieve wider electrochemical windows while maintaining high ionic conductivity. Harvard researchers have pioneered the use of computational screening methods combined with experimental validation to identify promising chloride-based materials with reduced susceptibility to redox side reactions. Their work has demonstrated that incorporating specific dopants and structural modifications can extend the stability window of chloride SSEs from typical 2.5-3V to over 4V, significantly improving compatibility with high-voltage cathode materials. Harvard has also developed innovative interface engineering strategies, including the application of artificial SEI (solid-electrolyte interphase) layers to mitigate interfacial reactions between chloride SSEs and electrode materials.
Strengths: Advanced computational screening capabilities allow rapid identification of promising materials; strong interdisciplinary approach combining materials science, electrochemistry, and computational modeling. Weaknesses: Some of their more advanced solutions remain at laboratory scale and face challenges in scaling to commercial production; interface engineering approaches may add complexity and cost to battery manufacturing processes.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an innovative approach to chloride solid-state electrolytes focusing on the critical challenge of electrochemical stability windows. Their technical solution involves a multi-layer electrolyte architecture where chloride-based materials are strategically combined with thin buffer layers to mitigate redox side reactions. CATL's research has demonstrated that incorporating specific halide mixtures (chloride-bromide-iodide) in precise ratios can extend the stability window to approximately 4.2V while maintaining ionic conductivities suitable for commercial applications. Their proprietary manufacturing process includes controlled atmosphere synthesis and processing to minimize contamination that could compromise electrochemical stability. CATL has also pioneered the use of specialized surface coatings on electrode materials that are specifically designed to be compatible with chloride electrolytes, reducing interfacial resistance and suppressing parasitic reactions. Their technology incorporates gradient-doped chloride SSEs where the concentration of stabilizing elements varies across the electrolyte thickness to optimize both stability and ionic transport.
Strengths: Extensive manufacturing infrastructure provides pathway to commercialization; integrated approach considering both electrolyte and electrode materials enables system-level optimization. Weaknesses: Their multi-layer approach increases manufacturing complexity; some of their more advanced formulations rely on costly rare earth dopants that may impact economic viability at scale.
Key Patents and Research on Chloride SSE Stability
Chlorine-Based Sodium Solid Electrolyte
PatentPendingUS20230115956A1
Innovation
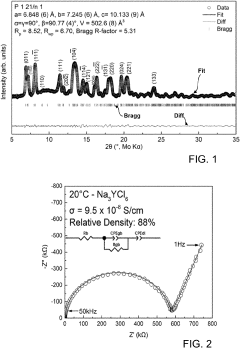
- Development of chloride-based sodium-ion conductors, specifically Na3-xY1-xZrxCl6 compounds, which enhance sodium diffusion and provide superior chemical and electrochemical stability with high voltage cathodes, preventing unwanted reactions and degradation, and are used in a cathode composite without protective coatings.
Conducting Polymer/Inorganic Hybrid Solid-State Electrolytes, Lithium Batteries Containing Same, and Production Processes
PatentPendingUS20230238571A1
Innovation
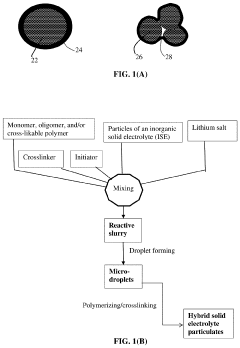
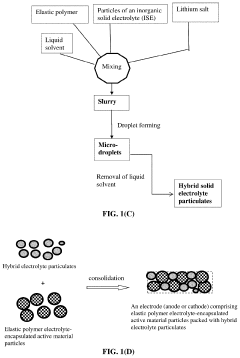
- A hybrid solid electrolyte system comprising inorganic solid electrolyte particles encapsulated in a conducting polymer shell, providing lithium-ion conductivity from 10−6 S/cm to 5×10−2 S/cm, electron conductivity, and a polymer-to-inorganic solid electrolyte ratio from 1/100 to 100/1, allowing for reduced electrolyte volume and improved electrode active material utilization.
Safety Standards for Solid-State Battery Technologies
The development of solid-state battery technologies necessitates comprehensive safety standards to address unique challenges posed by these advanced energy storage systems. Chloride solid-state electrolytes (SSEs) present particular safety concerns related to their electrochemical stability windows and potential redox side reactions that must be carefully regulated.
Current safety standards for chloride-based SSEs focus primarily on electrochemical stability parameters, requiring manufacturers to demonstrate stability windows compatible with intended electrode materials. These standards typically mandate stability testing across operational voltage ranges (2.0-4.5V vs. Li/Li+) to ensure minimal degradation during battery cycling.
Redox side reactions in chloride SSEs represent a critical safety concern addressed in emerging regulations. Standards now require quantification of reaction products formed at electrolyte-electrode interfaces, with particular emphasis on chlorine gas evolution potential. Maximum allowable chlorine emission rates have been established at 0.5 ppm under standard operating conditions.
Thermal runaway prevention standards have evolved specifically for chloride-based systems, with requirements for demonstrating stability at elevated temperatures (typically up to 150°C) and resistance to thermal decomposition. These standards include protocols for measuring heat generation during redox side reactions and establishing safety thresholds.
Interface stability requirements constitute another regulatory focus, with standards mandating characterization of solid-electrolyte interphase (SEI) formation in chloride systems. Manufacturers must demonstrate controlled SEI growth that maintains ionic conductivity while preventing dendrite formation or progressive impedance increases.
Testing protocols have been standardized to evaluate chloride SSE safety, including accelerated aging tests, electrochemical impedance spectroscopy requirements, and in-situ gas analysis during cycling. These protocols aim to identify potential failure modes before commercial deployment.
International harmonization efforts are underway to establish consistent safety standards across markets. The International Electrotechnical Commission (IEC) has formed a dedicated working group on solid-state battery safety, with specific provisions for halide-based electrolytes expected in upcoming standards revisions.
Regulatory compliance pathways have been established for manufacturers, requiring documentation of electrochemical stability windows, redox reaction mitigation strategies, and safety validation testing. These requirements are increasingly integrated into broader battery safety certification processes, ensuring chloride SSE technologies meet rigorous safety benchmarks before market entry.
Current safety standards for chloride-based SSEs focus primarily on electrochemical stability parameters, requiring manufacturers to demonstrate stability windows compatible with intended electrode materials. These standards typically mandate stability testing across operational voltage ranges (2.0-4.5V vs. Li/Li+) to ensure minimal degradation during battery cycling.
Redox side reactions in chloride SSEs represent a critical safety concern addressed in emerging regulations. Standards now require quantification of reaction products formed at electrolyte-electrode interfaces, with particular emphasis on chlorine gas evolution potential. Maximum allowable chlorine emission rates have been established at 0.5 ppm under standard operating conditions.
Thermal runaway prevention standards have evolved specifically for chloride-based systems, with requirements for demonstrating stability at elevated temperatures (typically up to 150°C) and resistance to thermal decomposition. These standards include protocols for measuring heat generation during redox side reactions and establishing safety thresholds.
Interface stability requirements constitute another regulatory focus, with standards mandating characterization of solid-electrolyte interphase (SEI) formation in chloride systems. Manufacturers must demonstrate controlled SEI growth that maintains ionic conductivity while preventing dendrite formation or progressive impedance increases.
Testing protocols have been standardized to evaluate chloride SSE safety, including accelerated aging tests, electrochemical impedance spectroscopy requirements, and in-situ gas analysis during cycling. These protocols aim to identify potential failure modes before commercial deployment.
International harmonization efforts are underway to establish consistent safety standards across markets. The International Electrotechnical Commission (IEC) has formed a dedicated working group on solid-state battery safety, with specific provisions for halide-based electrolytes expected in upcoming standards revisions.
Regulatory compliance pathways have been established for manufacturers, requiring documentation of electrochemical stability windows, redox reaction mitigation strategies, and safety validation testing. These requirements are increasingly integrated into broader battery safety certification processes, ensuring chloride SSE technologies meet rigorous safety benchmarks before market entry.
Environmental Impact of Chloride-based Electrolytes
The environmental implications of chloride-based solid-state electrolytes (SSEs) represent a critical dimension in evaluating their viability for next-generation energy storage systems. Unlike conventional liquid electrolytes containing volatile organic compounds, chloride-based SSEs offer reduced fire hazards and lower toxicity profiles during normal operation, contributing to safer energy storage solutions with minimized environmental risks.
However, the production processes for chloride-based electrolytes present notable environmental concerns. The extraction and processing of raw materials for chlorides, particularly lithium, sodium, and rare earth chlorides, involve energy-intensive mining operations that contribute to habitat disruption, water consumption, and carbon emissions. Manufacturing these electrolytes typically requires high-temperature synthesis methods, consuming significant energy and generating greenhouse gas emissions that impact climate change metrics.
Chloride-based electrolytes pose specific environmental challenges related to their chemical properties. The high reactivity of chloride ions with moisture can lead to the formation of hydrogen chloride gas when exposed to environmental humidity, creating potential air quality issues during improper handling or disposal. Additionally, the corrosive nature of chloride compounds may accelerate the degradation of containment materials, increasing the risk of leakage and environmental contamination over time.
End-of-life management presents particular challenges for chloride-based SSEs. Current recycling infrastructure is primarily designed for conventional battery technologies, leaving limited pathways for efficient recovery of materials from solid-state systems. The potential leaching of chloride compounds into soil and groundwater systems during improper disposal could adversely affect aquatic ecosystems and potentially contaminate drinking water sources, necessitating specialized waste management protocols.
Life cycle assessment (LCA) studies comparing chloride-based SSEs with conventional electrolytes show mixed results. While they demonstrate advantages in operational safety and reduced volatile organic compound emissions, the higher energy requirements for production and challenges in recycling may offset some environmental benefits. The environmental footprint varies significantly based on manufacturing methods, energy sources used in production, and end-of-life management approaches.
Regulatory frameworks governing the environmental aspects of chloride-based electrolytes are still evolving. Current regulations primarily focus on transportation safety and waste classification rather than comprehensive environmental impact management throughout the product lifecycle. As these technologies advance toward commercialization, more specific environmental guidelines and standards will likely emerge to address their unique characteristics and potential ecological impacts.
However, the production processes for chloride-based electrolytes present notable environmental concerns. The extraction and processing of raw materials for chlorides, particularly lithium, sodium, and rare earth chlorides, involve energy-intensive mining operations that contribute to habitat disruption, water consumption, and carbon emissions. Manufacturing these electrolytes typically requires high-temperature synthesis methods, consuming significant energy and generating greenhouse gas emissions that impact climate change metrics.
Chloride-based electrolytes pose specific environmental challenges related to their chemical properties. The high reactivity of chloride ions with moisture can lead to the formation of hydrogen chloride gas when exposed to environmental humidity, creating potential air quality issues during improper handling or disposal. Additionally, the corrosive nature of chloride compounds may accelerate the degradation of containment materials, increasing the risk of leakage and environmental contamination over time.
End-of-life management presents particular challenges for chloride-based SSEs. Current recycling infrastructure is primarily designed for conventional battery technologies, leaving limited pathways for efficient recovery of materials from solid-state systems. The potential leaching of chloride compounds into soil and groundwater systems during improper disposal could adversely affect aquatic ecosystems and potentially contaminate drinking water sources, necessitating specialized waste management protocols.
Life cycle assessment (LCA) studies comparing chloride-based SSEs with conventional electrolytes show mixed results. While they demonstrate advantages in operational safety and reduced volatile organic compound emissions, the higher energy requirements for production and challenges in recycling may offset some environmental benefits. The environmental footprint varies significantly based on manufacturing methods, energy sources used in production, and end-of-life management approaches.
Regulatory frameworks governing the environmental aspects of chloride-based electrolytes are still evolving. Current regulations primarily focus on transportation safety and waste classification rather than comprehensive environmental impact management throughout the product lifecycle. As these technologies advance toward commercialization, more specific environmental guidelines and standards will likely emerge to address their unique characteristics and potential ecological impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!