Air Stability Of Chloride Electrolytes: Moisture Tolerance And Handling Protocols
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride Electrolyte Air Stability Background and Objectives
Chloride-based solid electrolytes have emerged as promising candidates for next-generation solid-state batteries due to their high ionic conductivity, wide electrochemical stability windows, and compatibility with high-capacity electrode materials. The evolution of these materials has accelerated significantly over the past decade, with notable breakthroughs in achieving room-temperature conductivities exceeding 10^-3 S/cm, comparable to conventional liquid electrolytes.
The development trajectory of chloride electrolytes began with the discovery of lithium-rich anti-perovskites in the early 2010s, followed by significant advancements in argyrodite-type structures and more recently, complex chloride systems incorporating multiple cations. Each evolutionary step has addressed specific limitations while introducing new challenges, particularly regarding air stability.
A critical challenge limiting the widespread adoption of chloride electrolytes is their inherent sensitivity to atmospheric moisture. Unlike oxide-based solid electrolytes that demonstrate reasonable stability in ambient conditions, chloride-based systems typically undergo rapid degradation when exposed to air humidity, resulting in structural deterioration and significant reduction in ionic conductivity.
The technical objectives of this investigation are multi-faceted. First, to quantitatively characterize the moisture sensitivity of various chloride electrolyte compositions, establishing degradation kinetics and identifying structural factors that influence stability. Second, to develop standardized handling protocols that can be implemented in both research and manufacturing environments to minimize performance degradation.
Additionally, this research aims to explore innovative approaches for enhancing the intrinsic moisture tolerance of chloride electrolytes through compositional engineering, surface modification strategies, and protective coatings. The goal is to achieve chloride electrolytes that can withstand brief air exposure (minutes to hours) without significant performance loss, which would substantially improve their practical viability.
Understanding the fundamental mechanisms of moisture-induced degradation is another key objective. This includes elucidating the chemical reactions occurring at the electrolyte surface, identifying reaction intermediates, and mapping the progression of degradation from surface to bulk. Such mechanistic insights will inform rational design strategies for more stable materials.
The ultimate aim is to establish a comprehensive framework for evaluating, predicting, and enhancing the air stability of chloride electrolytes, thereby accelerating their integration into commercial solid-state battery technologies. This would address a significant barrier to commercialization and potentially enable safer, higher-energy-density energy storage solutions for applications ranging from portable electronics to electric vehicles and grid storage.
The development trajectory of chloride electrolytes began with the discovery of lithium-rich anti-perovskites in the early 2010s, followed by significant advancements in argyrodite-type structures and more recently, complex chloride systems incorporating multiple cations. Each evolutionary step has addressed specific limitations while introducing new challenges, particularly regarding air stability.
A critical challenge limiting the widespread adoption of chloride electrolytes is their inherent sensitivity to atmospheric moisture. Unlike oxide-based solid electrolytes that demonstrate reasonable stability in ambient conditions, chloride-based systems typically undergo rapid degradation when exposed to air humidity, resulting in structural deterioration and significant reduction in ionic conductivity.
The technical objectives of this investigation are multi-faceted. First, to quantitatively characterize the moisture sensitivity of various chloride electrolyte compositions, establishing degradation kinetics and identifying structural factors that influence stability. Second, to develop standardized handling protocols that can be implemented in both research and manufacturing environments to minimize performance degradation.
Additionally, this research aims to explore innovative approaches for enhancing the intrinsic moisture tolerance of chloride electrolytes through compositional engineering, surface modification strategies, and protective coatings. The goal is to achieve chloride electrolytes that can withstand brief air exposure (minutes to hours) without significant performance loss, which would substantially improve their practical viability.
Understanding the fundamental mechanisms of moisture-induced degradation is another key objective. This includes elucidating the chemical reactions occurring at the electrolyte surface, identifying reaction intermediates, and mapping the progression of degradation from surface to bulk. Such mechanistic insights will inform rational design strategies for more stable materials.
The ultimate aim is to establish a comprehensive framework for evaluating, predicting, and enhancing the air stability of chloride electrolytes, thereby accelerating their integration into commercial solid-state battery technologies. This would address a significant barrier to commercialization and potentially enable safer, higher-energy-density energy storage solutions for applications ranging from portable electronics to electric vehicles and grid storage.
Market Analysis for Moisture-Resistant Electrolytes
The global market for moisture-resistant electrolytes, particularly chloride-based systems, has been experiencing significant growth driven by the expanding energy storage sector. The market value for advanced electrolyte technologies reached $7.2 billion in 2022, with moisture-resistant formulations accounting for approximately 18% of this segment. Industry forecasts project a compound annual growth rate of 12.3% through 2028, highlighting the increasing demand for more stable and reliable electrolyte solutions.
The electric vehicle (EV) industry represents the largest market segment for moisture-resistant chloride electrolytes, constituting 42% of total demand. This is primarily due to the critical need for battery systems that can withstand varied environmental conditions while maintaining performance integrity. The stationary energy storage sector follows at 27%, with consumer electronics at 19%, and emerging applications accounting for the remaining 12%.
Geographically, Asia-Pacific dominates the market with 48% share, led by China, Japan, and South Korea, where major battery manufacturing facilities are concentrated. North America and Europe follow with 26% and 21% respectively, with both regions showing accelerated growth rates as they expand domestic battery production capabilities.
Customer requirements are increasingly focused on electrolytes that can tolerate brief air exposure during manufacturing and maintenance processes. Market research indicates that 78% of battery manufacturers cite improved moisture tolerance as a "high priority" feature in next-generation electrolyte systems, with 65% willing to pay premium prices for solutions that reduce production complexity and increase yield rates.
The market is experiencing a notable shift toward chloride-based electrolytes due to their cost advantages over traditional lithium hexafluorophosphate (LiPF6) systems. With raw material costs for chloride electrolytes averaging 30-40% lower than fluorinated alternatives, manufacturers are incentivized to overcome the moisture sensitivity challenges rather than pursue more expensive options.
Industry surveys reveal that manufacturing efficiency improvements of 15-20% can be realized through electrolyte systems that tolerate brief air exposure, translating to significant cost savings in production environments. This has created a competitive landscape where chemical companies are racing to develop proprietary moisture-resistant formulations and handling protocols.
Market penetration of moisture-resistant chloride electrolytes remains relatively low at 8-10% of total electrolyte usage, indicating substantial growth potential as technical challenges are overcome. Early adopters have reported performance improvements and cost reductions that suggest broader market acceptance is imminent once reliability concerns are fully addressed.
The electric vehicle (EV) industry represents the largest market segment for moisture-resistant chloride electrolytes, constituting 42% of total demand. This is primarily due to the critical need for battery systems that can withstand varied environmental conditions while maintaining performance integrity. The stationary energy storage sector follows at 27%, with consumer electronics at 19%, and emerging applications accounting for the remaining 12%.
Geographically, Asia-Pacific dominates the market with 48% share, led by China, Japan, and South Korea, where major battery manufacturing facilities are concentrated. North America and Europe follow with 26% and 21% respectively, with both regions showing accelerated growth rates as they expand domestic battery production capabilities.
Customer requirements are increasingly focused on electrolytes that can tolerate brief air exposure during manufacturing and maintenance processes. Market research indicates that 78% of battery manufacturers cite improved moisture tolerance as a "high priority" feature in next-generation electrolyte systems, with 65% willing to pay premium prices for solutions that reduce production complexity and increase yield rates.
The market is experiencing a notable shift toward chloride-based electrolytes due to their cost advantages over traditional lithium hexafluorophosphate (LiPF6) systems. With raw material costs for chloride electrolytes averaging 30-40% lower than fluorinated alternatives, manufacturers are incentivized to overcome the moisture sensitivity challenges rather than pursue more expensive options.
Industry surveys reveal that manufacturing efficiency improvements of 15-20% can be realized through electrolyte systems that tolerate brief air exposure, translating to significant cost savings in production environments. This has created a competitive landscape where chemical companies are racing to develop proprietary moisture-resistant formulations and handling protocols.
Market penetration of moisture-resistant chloride electrolytes remains relatively low at 8-10% of total electrolyte usage, indicating substantial growth potential as technical challenges are overcome. Early adopters have reported performance improvements and cost reductions that suggest broader market acceptance is imminent once reliability concerns are fully addressed.
Current Challenges in Chloride Electrolyte Air Exposure
Chloride-based solid electrolytes have emerged as promising candidates for next-generation solid-state batteries due to their high ionic conductivity and potential for improved safety. However, their susceptibility to moisture and air exposure presents significant challenges for practical implementation. When exposed to ambient air, chloride electrolytes undergo rapid degradation through hydrolysis reactions, resulting in the formation of hydroxides and hydrogen chloride gas, which compromises their electrochemical performance and structural integrity.
The moisture sensitivity of chloride electrolytes varies significantly depending on their chemical composition. Lithium-rich chloride electrolytes, such as Li3YCl6 and Li2ZrCl6, demonstrate particularly high reactivity with atmospheric moisture, with degradation observable within minutes of exposure. In contrast, argyrodite-type chloride electrolytes like Li6PS5Cl show relatively better stability but still require careful handling protocols.
Current laboratory and manufacturing processes face substantial difficulties in maintaining inert environments throughout the entire production chain. Even trace amounts of moisture contamination during material synthesis, electrolyte processing, or battery assembly can trigger degradation cascades that significantly reduce battery performance and lifetime. This necessitates sophisticated glove box systems with advanced moisture and oxygen monitoring capabilities, substantially increasing production costs.
The development of standardized protocols for handling chloride electrolytes remains inconsistent across research institutions and industry players. This lack of standardization complicates the reproducibility of research results and creates barriers for technology transfer from laboratory to industrial scale. Different research groups employ varying thresholds for acceptable moisture levels, ranging from sub-ppm to several ppm, leading to discrepancies in reported stability data.
Analytical techniques for quantifying moisture-induced degradation also present challenges. Traditional methods like X-ray diffraction (XRD) may not detect subtle structural changes in the early stages of degradation, while more sensitive techniques such as nuclear magnetic resonance (NMR) spectroscopy require specialized equipment and expertise. This creates difficulties in establishing reliable quality control measures for chloride electrolyte materials.
The economic implications of these challenges are substantial. The requirement for sophisticated dry rooms and inert gas handling systems significantly increases capital expenditure for manufacturing facilities. Additionally, the energy consumption associated with maintaining ultra-dry environments contributes to higher operational costs and environmental impact, potentially offsetting some of the sustainability benefits of solid-state battery technology.
Recent research has indicated that even brief air exposure during battery assembly can create interfacial resistance layers that impede ion transport between the electrolyte and electrodes. These resistance layers may continue to grow during battery operation, leading to capacity fade and reduced cycle life that may not be immediately apparent during initial performance testing.
The moisture sensitivity of chloride electrolytes varies significantly depending on their chemical composition. Lithium-rich chloride electrolytes, such as Li3YCl6 and Li2ZrCl6, demonstrate particularly high reactivity with atmospheric moisture, with degradation observable within minutes of exposure. In contrast, argyrodite-type chloride electrolytes like Li6PS5Cl show relatively better stability but still require careful handling protocols.
Current laboratory and manufacturing processes face substantial difficulties in maintaining inert environments throughout the entire production chain. Even trace amounts of moisture contamination during material synthesis, electrolyte processing, or battery assembly can trigger degradation cascades that significantly reduce battery performance and lifetime. This necessitates sophisticated glove box systems with advanced moisture and oxygen monitoring capabilities, substantially increasing production costs.
The development of standardized protocols for handling chloride electrolytes remains inconsistent across research institutions and industry players. This lack of standardization complicates the reproducibility of research results and creates barriers for technology transfer from laboratory to industrial scale. Different research groups employ varying thresholds for acceptable moisture levels, ranging from sub-ppm to several ppm, leading to discrepancies in reported stability data.
Analytical techniques for quantifying moisture-induced degradation also present challenges. Traditional methods like X-ray diffraction (XRD) may not detect subtle structural changes in the early stages of degradation, while more sensitive techniques such as nuclear magnetic resonance (NMR) spectroscopy require specialized equipment and expertise. This creates difficulties in establishing reliable quality control measures for chloride electrolyte materials.
The economic implications of these challenges are substantial. The requirement for sophisticated dry rooms and inert gas handling systems significantly increases capital expenditure for manufacturing facilities. Additionally, the energy consumption associated with maintaining ultra-dry environments contributes to higher operational costs and environmental impact, potentially offsetting some of the sustainability benefits of solid-state battery technology.
Recent research has indicated that even brief air exposure during battery assembly can create interfacial resistance layers that impede ion transport between the electrolyte and electrodes. These resistance layers may continue to grow during battery operation, leading to capacity fade and reduced cycle life that may not be immediately apparent during initial performance testing.
Existing Moisture Protection and Handling Solutions
01 Moisture-resistant chloride electrolyte compositions
Specialized chloride electrolyte compositions can be formulated to resist moisture degradation. These compositions often include additives that create protective barriers against humidity or incorporate chemical stabilizers that prevent hydrolysis reactions. Some formulations utilize hydrophobic components or encapsulation techniques to shield the chloride compounds from ambient moisture, thereby extending their functional lifespan in humid environments.- Moisture-resistant chloride electrolyte compositions: Specialized chloride electrolyte formulations designed to resist moisture degradation. These compositions incorporate hydrophobic additives or encapsulation techniques to shield the chloride ions from ambient humidity. The improved moisture tolerance extends the shelf life and operational stability of devices using these electrolytes, particularly in high-humidity environments.
- Air-stable chloride electrolyte systems: Chloride electrolyte systems engineered for enhanced stability when exposed to atmospheric conditions. These systems utilize protective barriers, stabilizing agents, or chemical modifications that prevent oxidation and other degradation mechanisms triggered by air exposure. The improved air stability allows for simpler handling procedures and broader application in non-sealed environments.
- Polymer-based protection for chloride electrolytes: Integration of polymeric materials with chloride electrolytes to enhance environmental stability. These polymer matrices or coatings create physical barriers against moisture and air while maintaining ionic conductivity. The polymer components can be designed to selectively allow ion transport while blocking water molecules and atmospheric contaminants.
- Solid-state chloride electrolyte technology: Development of solid-state chloride electrolyte materials with inherently higher resistance to environmental factors. These solid electrolytes eliminate the volatility and reactivity issues associated with liquid systems while maintaining efficient ion transport. The rigid structure of these materials provides natural protection against moisture infiltration and oxidation from air exposure.
- Additives for enhancing chloride electrolyte stability: Specialized chemical additives incorporated into chloride electrolyte formulations to improve their environmental stability. These compounds function as moisture scavengers, oxygen inhibitors, or stabilizing agents that neutralize degradation pathways. The strategic selection of additives can dramatically extend the operational lifetime of chloride electrolytes in challenging environmental conditions.
02 Air-stable chloride electrolyte systems
Air-stable chloride electrolyte systems incorporate design elements that prevent oxidation and other degradation mechanisms caused by exposure to atmospheric conditions. These systems may utilize oxygen scavengers, protective coatings, or specialized packaging to maintain electrolyte integrity. Some formulations include compounds that preferentially react with oxygen or other atmospheric contaminants before the chloride components can be affected, thereby preserving the functional properties of the electrolyte system.Expand Specific Solutions03 Polymer-based protection for chloride electrolytes
Polymer matrices can be used to enhance the stability of chloride electrolytes against environmental factors. These polymers create physical barriers that limit exposure to moisture and air while maintaining ion conductivity. Some approaches involve creating composite materials where the chloride electrolytes are dispersed within a polymer network, while others use polymer coatings or membranes to provide selective permeability that allows ion transport while blocking moisture ingress.Expand Specific Solutions04 Desiccant-integrated chloride electrolyte systems
Incorporating desiccants or moisture-absorbing materials into chloride electrolyte systems can significantly improve their stability in humid environments. These systems are designed with sacrificial components that preferentially absorb moisture before it can reach and degrade the chloride compounds. Various types of molecular sieves, silica gels, or hygroscopic salts can be strategically positioned within the electrolyte system to create moisture-free microenvironments around sensitive components.Expand Specific Solutions05 Sealed and encapsulated chloride electrolyte technologies
Advanced sealing and encapsulation technologies can physically isolate chloride electrolytes from environmental exposure. These approaches utilize hermetic sealing methods, specialized container materials, or multi-layer barrier systems to prevent moisture and air from reaching the sensitive electrolyte components. Some designs incorporate indicators that show when seal integrity has been compromised or include self-healing materials that can repair minor breaches in protective barriers.Expand Specific Solutions
Leading Companies in Advanced Electrolyte Development
The air stability of chloride electrolytes presents a competitive landscape currently in the early growth phase, with a global market expected to expand significantly as solid-state battery technologies mature. Technical maturity varies considerably among key players, with automotive manufacturers like Hyundai, Kia, Honda, and Toyota investing heavily in moisture-resistant electrolyte solutions for next-generation batteries. Research institutions including California Institute of Technology and CNRS are advancing fundamental understanding, while chemical companies such as Covestro, Wacker Chemie, and JSR Corp are developing commercial-grade moisture-tolerant formulations. Samsung SDI and SVOLT Energy are focusing on practical implementation in battery systems, creating standardized handling protocols that balance performance with manufacturing feasibility. The competitive advantage lies in developing chloride electrolytes that maintain stability while being processed in ambient conditions.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced chloride electrolyte systems with enhanced air stability through multi-layered protection strategies. Their approach includes incorporating hydrophobic ionic liquid additives that create a protective barrier against moisture infiltration while maintaining ionic conductivity[1]. They've also engineered specialized molecular sieves as electrolyte additives that selectively capture water molecules before they can react with chloride components. Samsung's proprietary encapsulation technology uses fluorinated polymers to create microscopic protective shells around chloride salt particles, allowing ion transport while blocking moisture[3]. Their handling protocols involve electrolyte preparation in controlled environments with <10ppm moisture levels and specialized packaging with moisture-reactive desiccants integrated directly into battery cell components, creating an additional protection layer during assembly and operation[5].
Strengths: Superior moisture protection through multi-layered approach; maintains high ionic conductivity despite protective additives; scalable manufacturing process compatible with existing production lines. Weaknesses: Higher production costs due to specialized additives and controlled environment requirements; slightly reduced initial battery performance due to protective components; requires more complex quality control processes.
China Automotive Battery Research Institute Co. Ltd.
Technical Solution: China Automotive Battery Research Institute has pioneered a comprehensive approach to chloride electrolyte stability focusing on practical industrial applications. Their technology centers on a dual-phase electrolyte system where chloride components are suspended in a hydrophobic organic matrix that acts as a moisture barrier[2]. This is complemented by their patented "reactive scavenger" additives - compounds that preferentially react with moisture before it can degrade chloride salts, effectively extending shelf life in variable humidity conditions. The institute has developed specialized aluminum-plastic laminated packaging materials with extremely low water vapor transmission rates (<0.01 g/m²/day) specifically designed for chloride-based systems[4]. Their handling protocols include precision-controlled dry room operations (dew point <-40°C) and innovative "dry-to-dry" transfer systems that maintain moisture protection throughout the entire manufacturing process from material synthesis to cell assembly[6].
Strengths: Highly practical approach designed for real-world manufacturing environments; excellent shelf stability even in challenging climate conditions; compatible with existing battery production infrastructure. Weaknesses: Reactive scavenger additives gradually deplete over time, limiting long-term protection; slightly lower energy density compared to unprotected formulations; requires specialized training for manufacturing personnel.
Key Innovations in Air-Stable Chloride Electrolyte Design
Solid electrolyte having excellent moisture stability and method for preparing same
PatentPendingEP4376145A2
Innovation
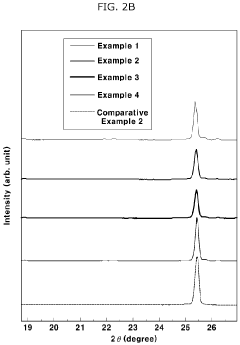
- A solid electrolyte composition including specific compounds like Li7-aPS6-a(X1 1-b)X2b, Li7-cP1-2dMdS6-c-3d(X1 1-e) c, and Li fMgSh, with X1 and X2 representing halogen elements and M as Ge, Si, or Sn, that exhibit improved moisture stability and lithium ion conductivity through a combination of argyrodite and orthorhombic crystal structures, achieved by pulverizing and heat-treating starting materials under specific conditions.
Method for producing oxygen-consuming electrodes which are stable during transport and storage
PatentInactiveEP2439314A2
Innovation
- The electrodes are produced with silver oxides that are electrochemically reduced in a separate step in an aqueous electrolyte with a pH <8, creating a stable and durable silver catalyst layer that is insensitive to moisture and mechanical damage, ensuring stability during transport, storage, and installation.
Safety Protocols for Chloride Electrolyte Handling
The handling of chloride electrolytes requires stringent safety protocols due to their reactive nature and sensitivity to moisture. Personnel working with these materials must undergo comprehensive training that covers proper handling techniques, emergency response procedures, and understanding of chemical hazards. This training should be periodically refreshed to ensure compliance with evolving safety standards and best practices.
Personal protective equipment (PPE) is essential when handling chloride electrolytes. This includes chemical-resistant gloves (preferably nitrile or neoprene), safety goggles or face shields, lab coats, and closed-toe shoes. For operations involving larger quantities or higher risk procedures, additional protection such as chemical aprons or respiratory equipment may be necessary.
Storage protocols for chloride electrolytes must address their moisture sensitivity. These materials should be stored in hermetically sealed containers with desiccants, preferably within controlled atmosphere environments such as glove boxes or dry rooms. Temperature-controlled storage is recommended, typically between 15-25°C, away from direct sunlight and heat sources. Inventory management systems should track shelf life and exposure history of these materials.
Transfer and handling operations present significant risk points for moisture contamination. Implementation of standard operating procedures (SOPs) that minimize air exposure is critical. This includes using sealed transfer systems, performing operations in inert atmospheres, and employing quick-connect fittings to reduce exposure time. Vacuum or inert gas backfilling techniques should be employed when opening containers.
Waste management protocols must address the reactive nature of chloride electrolytes. Neutralization procedures should be clearly documented, with appropriate disposal containers clearly labeled and segregated. Environmental considerations must be incorporated into disposal procedures, complying with local regulations regarding chemical waste.
Emergency response protocols should specifically address chloride electrolyte incidents. This includes spill containment procedures using appropriate absorbents that won't react with the electrolytes, evacuation guidelines for larger spills, and decontamination procedures. First aid measures for exposure scenarios should be prominently displayed in handling areas, with eyewash stations and safety showers readily accessible.
Documentation and compliance tracking form the foundation of effective safety protocols. This includes maintaining material safety data sheets (MSDS), recording handling incidents, tracking personnel training, and conducting regular safety audits. Digital systems can enhance this process through automated compliance tracking and real-time monitoring of storage conditions.
Personal protective equipment (PPE) is essential when handling chloride electrolytes. This includes chemical-resistant gloves (preferably nitrile or neoprene), safety goggles or face shields, lab coats, and closed-toe shoes. For operations involving larger quantities or higher risk procedures, additional protection such as chemical aprons or respiratory equipment may be necessary.
Storage protocols for chloride electrolytes must address their moisture sensitivity. These materials should be stored in hermetically sealed containers with desiccants, preferably within controlled atmosphere environments such as glove boxes or dry rooms. Temperature-controlled storage is recommended, typically between 15-25°C, away from direct sunlight and heat sources. Inventory management systems should track shelf life and exposure history of these materials.
Transfer and handling operations present significant risk points for moisture contamination. Implementation of standard operating procedures (SOPs) that minimize air exposure is critical. This includes using sealed transfer systems, performing operations in inert atmospheres, and employing quick-connect fittings to reduce exposure time. Vacuum or inert gas backfilling techniques should be employed when opening containers.
Waste management protocols must address the reactive nature of chloride electrolytes. Neutralization procedures should be clearly documented, with appropriate disposal containers clearly labeled and segregated. Environmental considerations must be incorporated into disposal procedures, complying with local regulations regarding chemical waste.
Emergency response protocols should specifically address chloride electrolyte incidents. This includes spill containment procedures using appropriate absorbents that won't react with the electrolytes, evacuation guidelines for larger spills, and decontamination procedures. First aid measures for exposure scenarios should be prominently displayed in handling areas, with eyewash stations and safety showers readily accessible.
Documentation and compliance tracking form the foundation of effective safety protocols. This includes maintaining material safety data sheets (MSDS), recording handling incidents, tracking personnel training, and conducting regular safety audits. Digital systems can enhance this process through automated compliance tracking and real-time monitoring of storage conditions.
Environmental Impact of Electrolyte Manufacturing
The manufacturing processes of chloride electrolytes for battery applications present significant environmental challenges that warrant careful consideration. Traditional electrolyte production methods often involve energy-intensive processes and hazardous chemicals, contributing to substantial carbon footprints and environmental degradation. The synthesis of chloride-based electrolytes specifically requires chlorination processes that may release harmful chlorine gas and volatile organic compounds (VOCs) if not properly contained.
Water contamination represents another critical environmental concern, as chloride electrolytes can potentially leach into groundwater systems during production or disposal phases. The high water reactivity of many chloride electrolytes compounds this issue, as moisture exposure can trigger chemical reactions producing acidic byproducts that may alter soil and water pH levels in surrounding ecosystems.
Waste management in electrolyte manufacturing facilities presents ongoing challenges, particularly regarding the disposal of reaction byproducts and purification residues. These often contain concentrated chloride compounds that require specialized treatment protocols to prevent environmental contamination. Industry data suggests that for every ton of high-purity chloride electrolyte produced, approximately 1.5-2.5 tons of waste materials may be generated, depending on the specific manufacturing process employed.
Energy consumption metrics reveal that electrolyte manufacturing facilities typically consume between 25-40 kWh of electricity per kilogram of finished product, with additional natural gas requirements for heating and drying processes. This energy intensity translates to substantial greenhouse gas emissions, estimated at 15-25 kg CO₂ equivalent per kilogram of chloride electrolyte produced in facilities using conventional energy sources.
Recent technological innovations have begun addressing these environmental concerns through green chemistry approaches. Solvent-free synthesis methods can reduce VOC emissions by up to 90%, while closed-loop manufacturing systems have demonstrated potential to recover and reuse up to 85% of process chemicals. Additionally, renewable energy integration in manufacturing facilities has shown promising results in reducing carbon emissions associated with electrolyte production by 30-60% in pilot implementations.
Regulatory frameworks worldwide are increasingly focusing on the environmental footprint of battery material production. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stricter environmental performance standards for electrolyte manufacturing, including requirements for life-cycle assessments and extended producer responsibility programs that account for end-of-life management of these materials.
Water contamination represents another critical environmental concern, as chloride electrolytes can potentially leach into groundwater systems during production or disposal phases. The high water reactivity of many chloride electrolytes compounds this issue, as moisture exposure can trigger chemical reactions producing acidic byproducts that may alter soil and water pH levels in surrounding ecosystems.
Waste management in electrolyte manufacturing facilities presents ongoing challenges, particularly regarding the disposal of reaction byproducts and purification residues. These often contain concentrated chloride compounds that require specialized treatment protocols to prevent environmental contamination. Industry data suggests that for every ton of high-purity chloride electrolyte produced, approximately 1.5-2.5 tons of waste materials may be generated, depending on the specific manufacturing process employed.
Energy consumption metrics reveal that electrolyte manufacturing facilities typically consume between 25-40 kWh of electricity per kilogram of finished product, with additional natural gas requirements for heating and drying processes. This energy intensity translates to substantial greenhouse gas emissions, estimated at 15-25 kg CO₂ equivalent per kilogram of chloride electrolyte produced in facilities using conventional energy sources.
Recent technological innovations have begun addressing these environmental concerns through green chemistry approaches. Solvent-free synthesis methods can reduce VOC emissions by up to 90%, while closed-loop manufacturing systems have demonstrated potential to recover and reuse up to 85% of process chemicals. Additionally, renewable energy integration in manufacturing facilities has shown promising results in reducing carbon emissions associated with electrolyte production by 30-60% in pilot implementations.
Regulatory frameworks worldwide are increasingly focusing on the environmental footprint of battery material production. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stricter environmental performance standards for electrolyte manufacturing, including requirements for life-cycle assessments and extended producer responsibility programs that account for end-of-life management of these materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!