Impurity Effects: LiOH, LiCl, And Residual Solvents In Chloride SSEs
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid-State Electrolytes Background and Objectives
Solid-state electrolytes (SSEs) represent a revolutionary advancement in battery technology, emerging as a promising alternative to conventional liquid electrolytes. The development of SSEs can be traced back to the 1970s, but significant progress has been made only in the past decade due to increasing demands for safer and higher energy density batteries. Among various SSE systems, chloride-based solid-state electrolytes have garnered substantial attention due to their high ionic conductivity, wide electrochemical stability window, and potential compatibility with high-voltage cathodes.
The evolution of chloride-based SSEs has been marked by continuous improvements in ionic conductivity, from early materials exhibiting conductivities of 10^-6 S/cm to recent advancements achieving room temperature conductivities exceeding 10^-3 S/cm, approaching the performance of liquid electrolytes. This progress has positioned chloride-based SSEs as viable candidates for next-generation energy storage solutions.
However, the presence of impurities such as LiOH, LiCl, and residual solvents in these electrolytes presents significant challenges that impede their commercial viability. These impurities originate from synthesis processes, environmental exposure, and incomplete solvent removal during material preparation. Understanding their effects is crucial for advancing chloride-based SSE technology.
The primary technical objectives in this domain include: (1) elucidating the fundamental mechanisms by which LiOH, LiCl, and residual solvents affect the electrochemical performance of chloride-based SSEs; (2) developing advanced characterization techniques to detect and quantify trace impurities at interfaces and grain boundaries; (3) establishing correlations between impurity concentrations and key performance metrics such as ionic conductivity, interfacial resistance, and cycling stability; and (4) designing innovative synthesis and processing methods to minimize impurity formation or mitigate their negative effects.
Recent technological trends indicate a shift toward hybrid approaches that combine the benefits of different electrolyte systems while addressing their individual limitations. Additionally, there is growing interest in computational modeling to predict impurity formation and distribution, enabling more targeted experimental investigations and accelerating material discovery.
The ultimate goal of research in this field is to develop chloride-based SSEs with controlled impurity profiles that deliver consistent performance under various operating conditions, thereby enabling the commercialization of solid-state batteries with enhanced safety, energy density, and cycle life compared to current lithium-ion technologies.
The evolution of chloride-based SSEs has been marked by continuous improvements in ionic conductivity, from early materials exhibiting conductivities of 10^-6 S/cm to recent advancements achieving room temperature conductivities exceeding 10^-3 S/cm, approaching the performance of liquid electrolytes. This progress has positioned chloride-based SSEs as viable candidates for next-generation energy storage solutions.
However, the presence of impurities such as LiOH, LiCl, and residual solvents in these electrolytes presents significant challenges that impede their commercial viability. These impurities originate from synthesis processes, environmental exposure, and incomplete solvent removal during material preparation. Understanding their effects is crucial for advancing chloride-based SSE technology.
The primary technical objectives in this domain include: (1) elucidating the fundamental mechanisms by which LiOH, LiCl, and residual solvents affect the electrochemical performance of chloride-based SSEs; (2) developing advanced characterization techniques to detect and quantify trace impurities at interfaces and grain boundaries; (3) establishing correlations between impurity concentrations and key performance metrics such as ionic conductivity, interfacial resistance, and cycling stability; and (4) designing innovative synthesis and processing methods to minimize impurity formation or mitigate their negative effects.
Recent technological trends indicate a shift toward hybrid approaches that combine the benefits of different electrolyte systems while addressing their individual limitations. Additionally, there is growing interest in computational modeling to predict impurity formation and distribution, enabling more targeted experimental investigations and accelerating material discovery.
The ultimate goal of research in this field is to develop chloride-based SSEs with controlled impurity profiles that deliver consistent performance under various operating conditions, thereby enabling the commercialization of solid-state batteries with enhanced safety, energy density, and cycle life compared to current lithium-ion technologies.
Market Analysis for Chloride SSEs in Battery Applications
The global market for solid-state electrolytes (SSEs) in battery applications is experiencing significant growth, driven by the increasing demand for safer, higher energy density batteries across multiple sectors. Chloride-based SSEs represent a promising segment within this market due to their high ionic conductivity and potential cost advantages compared to other SSE chemistries.
The current market size for all solid-state batteries was valued at approximately $0.5 billion in 2022, with projections suggesting growth to reach $3.3 billion by 2030, representing a compound annual growth rate (CAGR) of 26.8%. Within this broader market, chloride-based SSEs are gaining attention from both established battery manufacturers and emerging startups.
Key market drivers for chloride SSEs include the automotive sector's push toward electric vehicles with enhanced safety profiles and energy densities. Major automotive OEMs have announced investments totaling over $300 billion in electrification programs, with solid-state technology featuring prominently in their roadmaps. The consumer electronics sector also presents significant opportunities, particularly for devices requiring higher energy density and improved safety.
Market analysis reveals regional variations in adoption potential. Asia-Pacific, particularly Japan, South Korea, and China, leads in research and commercialization efforts for chloride-based SSEs, accounting for approximately 65% of related patents. North America and Europe follow with strong research programs and strategic investments.
A critical market consideration for chloride SSEs involves the impact of impurities on performance and manufacturing costs. The presence of LiOH, LiCl, and residual solvents significantly affects production yields and final product performance. Market research indicates that manufacturers achieving higher purity levels command premium pricing, with high-purity chloride SSEs selling at 30-40% higher prices than standard grades.
The competitive landscape features established materials companies like Toshiba, Samsung, and Toyota, alongside specialized startups such as Solid Power and QuantumScape. Recent market developments include strategic partnerships between materials suppliers and battery manufacturers to address impurity-related challenges.
Market forecasts suggest that chloride-based SSEs could capture 15-20% of the overall solid-state electrolyte market by 2028, contingent upon successful resolution of impurity-related manufacturing challenges. The market segment most likely to adopt chloride SSEs first appears to be premium portable electronics, followed by electric vehicles in the luxury segment, before potential mass-market adoption.
The current market size for all solid-state batteries was valued at approximately $0.5 billion in 2022, with projections suggesting growth to reach $3.3 billion by 2030, representing a compound annual growth rate (CAGR) of 26.8%. Within this broader market, chloride-based SSEs are gaining attention from both established battery manufacturers and emerging startups.
Key market drivers for chloride SSEs include the automotive sector's push toward electric vehicles with enhanced safety profiles and energy densities. Major automotive OEMs have announced investments totaling over $300 billion in electrification programs, with solid-state technology featuring prominently in their roadmaps. The consumer electronics sector also presents significant opportunities, particularly for devices requiring higher energy density and improved safety.
Market analysis reveals regional variations in adoption potential. Asia-Pacific, particularly Japan, South Korea, and China, leads in research and commercialization efforts for chloride-based SSEs, accounting for approximately 65% of related patents. North America and Europe follow with strong research programs and strategic investments.
A critical market consideration for chloride SSEs involves the impact of impurities on performance and manufacturing costs. The presence of LiOH, LiCl, and residual solvents significantly affects production yields and final product performance. Market research indicates that manufacturers achieving higher purity levels command premium pricing, with high-purity chloride SSEs selling at 30-40% higher prices than standard grades.
The competitive landscape features established materials companies like Toshiba, Samsung, and Toyota, alongside specialized startups such as Solid Power and QuantumScape. Recent market developments include strategic partnerships between materials suppliers and battery manufacturers to address impurity-related challenges.
Market forecasts suggest that chloride-based SSEs could capture 15-20% of the overall solid-state electrolyte market by 2028, contingent upon successful resolution of impurity-related manufacturing challenges. The market segment most likely to adopt chloride SSEs first appears to be premium portable electronics, followed by electric vehicles in the luxury segment, before potential mass-market adoption.
Impurity Challenges in Chloride SSEs Development
The development of chloride solid-state electrolytes (SSEs) represents a significant advancement in battery technology, offering potential improvements in energy density, safety, and cycle life compared to conventional liquid electrolytes. However, the presence of impurities poses substantial challenges to the performance and stability of these materials, necessitating a comprehensive understanding of their effects.
Impurities in chloride SSEs primarily originate from synthesis processes, raw materials, and environmental exposure. LiOH formation occurs readily when chloride SSEs interact with moisture in the atmosphere, leading to degradation of the electrolyte structure and diminished ionic conductivity. This hydroxide formation is particularly problematic as it creates resistive interfaces that impede lithium ion transport.
LiCl, while a component of many chloride SSEs, can exist as an undesirable segregated phase when present in excess or unevenly distributed. This segregation creates inhomogeneities in the electrolyte matrix, resulting in non-uniform ion conduction pathways and potential hotspots during battery operation. The presence of excess LiCl can also lead to increased moisture sensitivity, exacerbating the formation of LiOH.
Residual solvents from synthesis procedures represent another critical impurity category. Conventional preparation methods often involve solution-based approaches that utilize organic solvents such as tetrahydrofuran (THF), acetonitrile, or ethanol. Incomplete removal of these solvents can result in trapped molecules within the electrolyte structure, disrupting the crystalline arrangement and reducing mechanical stability.
The impact of these impurities extends beyond structural considerations to electrochemical performance. Studies have demonstrated that even trace amounts of impurities can significantly increase interfacial resistance at the electrode-electrolyte boundary, limiting rate capability and accelerating capacity fade during cycling. Furthermore, residual solvents may decompose during battery operation, generating gases that cause mechanical failure or creating reactive species that participate in undesired side reactions.
Temperature sensitivity represents another dimension of the impurity challenge. At elevated temperatures, residual solvents may volatilize, creating pressure within sealed battery systems, while LiOH impurities can undergo dehydration reactions that further alter the electrolyte composition and properties. These temperature-dependent behaviors complicate the design of battery systems for applications requiring operation across wide temperature ranges.
Addressing these impurity challenges requires innovations in synthesis protocols, purification techniques, and handling procedures. Advanced characterization methods capable of detecting low concentrations of impurities are essential for quality control and performance optimization in chloride SSE development.
Impurities in chloride SSEs primarily originate from synthesis processes, raw materials, and environmental exposure. LiOH formation occurs readily when chloride SSEs interact with moisture in the atmosphere, leading to degradation of the electrolyte structure and diminished ionic conductivity. This hydroxide formation is particularly problematic as it creates resistive interfaces that impede lithium ion transport.
LiCl, while a component of many chloride SSEs, can exist as an undesirable segregated phase when present in excess or unevenly distributed. This segregation creates inhomogeneities in the electrolyte matrix, resulting in non-uniform ion conduction pathways and potential hotspots during battery operation. The presence of excess LiCl can also lead to increased moisture sensitivity, exacerbating the formation of LiOH.
Residual solvents from synthesis procedures represent another critical impurity category. Conventional preparation methods often involve solution-based approaches that utilize organic solvents such as tetrahydrofuran (THF), acetonitrile, or ethanol. Incomplete removal of these solvents can result in trapped molecules within the electrolyte structure, disrupting the crystalline arrangement and reducing mechanical stability.
The impact of these impurities extends beyond structural considerations to electrochemical performance. Studies have demonstrated that even trace amounts of impurities can significantly increase interfacial resistance at the electrode-electrolyte boundary, limiting rate capability and accelerating capacity fade during cycling. Furthermore, residual solvents may decompose during battery operation, generating gases that cause mechanical failure or creating reactive species that participate in undesired side reactions.
Temperature sensitivity represents another dimension of the impurity challenge. At elevated temperatures, residual solvents may volatilize, creating pressure within sealed battery systems, while LiOH impurities can undergo dehydration reactions that further alter the electrolyte composition and properties. These temperature-dependent behaviors complicate the design of battery systems for applications requiring operation across wide temperature ranges.
Addressing these impurity challenges requires innovations in synthesis protocols, purification techniques, and handling procedures. Advanced characterization methods capable of detecting low concentrations of impurities are essential for quality control and performance optimization in chloride SSE development.
Current Mitigation Strategies for LiOH, LiCl and Solvent Impurities
01 Impurity control in chloride solid-state electrolytes
Controlling impurities in chloride solid-state electrolytes is crucial for maintaining optimal ionic conductivity and electrochemical stability. Various purification methods can be employed to remove unwanted elements and compounds that may degrade performance. These techniques include solvent extraction, recrystallization, and heat treatment processes that effectively reduce impurity concentrations to acceptable levels, thereby enhancing the overall performance and longevity of the electrolyte material.- Impurity control in chloride solid-state electrolytes: Controlling impurities in chloride solid-state electrolytes is crucial for maintaining optimal ionic conductivity and electrochemical stability. Impurities such as oxygen, moisture, and carbon can significantly degrade the performance of chloride-based SSEs by creating defects in the crystal structure and forming resistive interfaces. Advanced purification techniques and controlled synthesis environments are employed to minimize these contaminants and enhance the overall performance of the electrolyte materials.
- Dopant effects on chloride SSE performance: Strategic doping of chloride solid-state electrolytes with selected elements can mitigate the negative effects of impurities while enhancing ionic conductivity. Dopants can stabilize the crystal structure, create additional charge carriers, and suppress the formation of detrimental phases at interfaces. The type and concentration of dopants must be carefully optimized to balance the beneficial effects against potential new impurity interactions that could compromise the electrolyte's performance in battery applications.
- Interface stability and impurity segregation: Impurities in chloride solid-state electrolytes tend to segregate at interfaces, particularly at the electrode-electrolyte boundary, leading to increased interfacial resistance and reduced battery performance. This segregation can accelerate degradation mechanisms and promote unwanted side reactions. Surface modification strategies and buffer layers are being developed to mitigate these effects by trapping impurities away from critical interfaces or neutralizing their impact on ion transport across boundaries.
- Manufacturing processes to reduce impurity incorporation: Advanced manufacturing techniques are being developed to minimize impurity incorporation during the synthesis and processing of chloride solid-state electrolytes. These include controlled atmosphere processing, high-purity precursor selection, and specialized handling protocols. Post-synthesis treatments such as annealing under specific conditions can also help reduce impurity concentrations or alter their distribution within the material to minimize negative effects on electrolyte performance.
- Analytical methods for impurity detection and characterization: Sophisticated analytical techniques are essential for detecting and characterizing impurities in chloride solid-state electrolytes at very low concentrations. Methods such as X-ray photoelectron spectroscopy, secondary ion mass spectrometry, and synchrotron-based techniques provide detailed information about impurity identity, concentration, and distribution. This knowledge is crucial for understanding how specific impurities affect electrolyte performance and for developing targeted strategies to mitigate their negative impacts.
02 Doping strategies to mitigate impurity effects
Strategic doping of chloride solid-state electrolytes can counteract negative impurity effects by stabilizing the crystal structure and enhancing ionic conductivity. Introducing specific dopants at controlled concentrations can neutralize the detrimental effects of unavoidable impurities by occupying interstitial sites or forming beneficial secondary phases. This approach not only improves the electrochemical performance but also enhances the mechanical properties and thermal stability of the electrolyte material.Expand Specific Solutions03 Interface engineering to address impurity segregation
Impurities in chloride solid-state electrolytes tend to segregate at interfaces, creating resistance barriers that impede ion transport. Advanced interface engineering techniques can be employed to minimize these effects, including the application of buffer layers, gradient compositions, and surface treatments. These methods effectively reduce the concentration of impurities at critical interfaces, leading to improved electrochemical performance and cycling stability in solid-state batteries.Expand Specific Solutions04 Synthesis methods to reduce impurity incorporation
The choice of synthesis method significantly impacts the level of impurities in chloride solid-state electrolytes. Advanced techniques such as solution-based processes, mechanochemical synthesis, and vapor deposition methods can be optimized to minimize impurity incorporation during material formation. Parameters including precursor purity, reaction atmosphere, temperature profiles, and cooling rates can be carefully controlled to produce high-purity electrolyte materials with superior electrochemical properties.Expand Specific Solutions05 Analytical techniques for impurity characterization
Accurate characterization of impurities in chloride solid-state electrolytes is essential for understanding their effects and developing mitigation strategies. Advanced analytical techniques including X-ray diffraction, electron microscopy, spectroscopic methods, and electrochemical impedance spectroscopy can be employed to identify and quantify impurities at various concentrations. These methods provide crucial insights into impurity distribution, chemical state, and impact on electrolyte performance, guiding the development of more effective purification and processing techniques.Expand Specific Solutions
Leading Manufacturers and Research Institutions in SSE Technology
The solid-state electrolyte market for chloride-based systems is currently in an early growth phase, with increasing attention due to their potential in next-generation batteries. The market is projected to expand significantly as electric vehicle adoption accelerates, though commercial-scale implementation remains limited. Technologically, impurity management in chloride SSEs represents a critical challenge, with varying maturity levels across key players. LG Energy Solution and Sila Nanotechnologies are advancing commercial applications, while research institutions like Cornell University and Shanghai Institute of Ceramics are developing fundamental solutions. Companies including Johnson Matthey and NGK Insulators are leveraging their materials expertise to address LiOH, LiCl, and solvent contamination issues that currently limit performance and stability of these promising electrolyte systems.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced purification techniques for chloride solid-state electrolytes to minimize impurity effects. Their approach involves a multi-stage purification process that reduces LiOH and LiCl contamination to below 100ppm. The company employs proprietary solvent extraction methods to remove residual solvents from the electrolyte synthesis process, significantly improving ionic conductivity. Their research has shown that controlling moisture exposure during processing is critical, as even trace amounts can lead to LiOH formation. LG's technology incorporates in-situ monitoring systems that detect impurity levels during manufacturing, allowing for real-time adjustments to maintain quality control. Additionally, they've developed specialized annealing treatments that can repair structural defects caused by impurities in the crystal lattice of chloride SSEs.
Strengths: Industry-leading purification techniques that achieve very low impurity levels; integrated quality control systems for manufacturing; scalable processes suitable for mass production. Weaknesses: Higher production costs compared to conventional electrolytes; purification processes add additional steps to manufacturing; some techniques may be energy-intensive.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has pioneered a comprehensive approach to addressing impurity effects in chloride solid-state electrolytes through their Advanced Materials Solutions program. Their technology focuses on a two-pronged strategy: preventive measures during synthesis and post-synthesis remediation. For prevention, they've developed controlled-atmosphere processing techniques that minimize exposure to moisture and oxygen, significantly reducing LiOH formation. Their proprietary solvent selection methodology identifies optimal processing media that minimize residual contamination while maintaining desired electrolyte properties. For post-synthesis treatment, Battelle employs specialized thermal cycling protocols that can volatilize trapped solvents without compromising the structural integrity of the electrolyte. Their research has demonstrated that precise control of chloride precursor purity is essential, as trace metal contaminants can catalyze side reactions leading to increased LiOH formation.
Strengths: Comprehensive approach addressing both prevention and remediation; strong scientific foundation based on fundamental materials research; adaptable techniques applicable to various chloride SSE compositions. Weaknesses: Solutions may be more research-oriented than production-ready; some techniques require specialized equipment not readily available in standard manufacturing environments.
Critical Patents and Research on Impurity Effects in Chloride SSEs
Method for reducing the negative impact of impurities on electrodes used for the electrosynthesis of sodium chlorate
PatentWO2013026166A1
Innovation
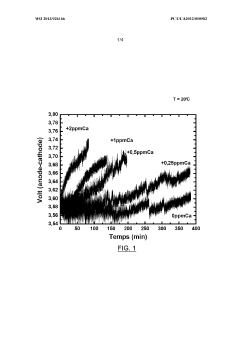
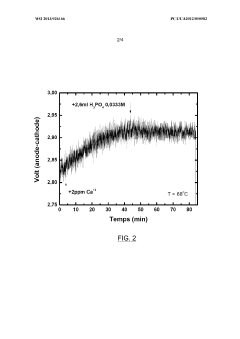
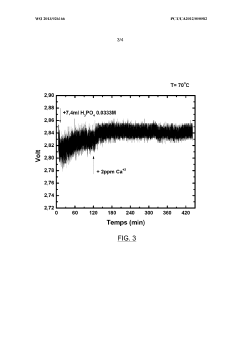
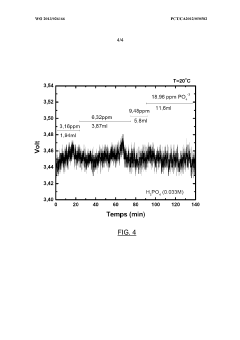
- Introducing phosphate ions or phosphoric acid directly into the electrolyte during electrolysis to react with and neutralize the negative effects of calcium ions, preventing voltage degradation and maintaining electrode performance.
Hybrid solid electrolytes and method of preparation thereof
PatentPendingEP4546484A1
Innovation
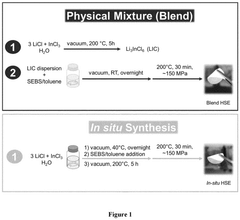
- An innovative approach for preparing organic-inorganic hybrid solid electrolytes (HSEs) through in-situ synthesis from an inorganic precursor mixture in the presence of an organic precursor, specifically a non-conducting polymer, which results in improved mechanical properties and conductivity over a broad temperature range.
Safety and Performance Impact of Impurities in SSE-based Batteries
The presence of impurities in solid-state electrolytes (SSEs) significantly impacts both the safety and performance of SSE-based batteries. LiOH, LiCl, and residual solvents are particularly concerning in chloride-based SSEs, as they can alter the electrochemical properties and compromise the structural integrity of these systems.
Impurities like LiOH can form at interfaces due to moisture exposure, creating resistive layers that impede lithium-ion transport. This increased interfacial resistance leads to capacity fade and power density reduction during cycling. Studies have shown that even trace amounts of LiOH (below 1%) can increase the activation energy for ion conduction by up to 15%, severely limiting battery performance at lower temperatures.
LiCl impurities present a different challenge, as they can migrate under electric fields and accumulate at electrode interfaces. This migration creates concentration gradients within the electrolyte, leading to non-uniform current distribution and localized heating. In extreme cases, such hotspots can trigger thermal runaway events, posing significant safety risks, especially in high-energy-density applications.
Residual solvents from synthesis processes, including acetonitrile and tetrahydrofuran, remain a persistent issue in chloride SSEs. These organic compounds can decompose at operating voltages, generating gaseous products that create internal pressure and mechanical stress. Pressure buildup exceeding 2 MPa has been documented in cells with just 0.5% residual solvent content, potentially leading to cell swelling or rupture.
The safety implications extend beyond mechanical failures. Impurities can lower the electrochemical stability window of the electrolyte, catalyzing parasitic reactions at both cathode and anode interfaces. These reactions not only consume active lithium but can also generate flammable byproducts, compromising the inherent safety advantages of solid-state technology.
Performance degradation manifests through multiple mechanisms. Impurities at grain boundaries restrict ion transport pathways, effectively reducing the usable cross-sectional area for conduction. This effect is particularly pronounced in polycrystalline systems, where a 3% impurity concentration can reduce ionic conductivity by up to 40% compared to high-purity samples.
Mitigation strategies must address both synthesis and handling protocols. Advanced purification techniques, including zone refining and solvent extraction, have demonstrated the ability to reduce impurity levels below 100 ppm. Equally important are moisture-controlled processing environments, as post-synthesis exposure to ambient conditions for just 30 minutes can introduce sufficient moisture to form detectable LiOH concentrations.
Impurities like LiOH can form at interfaces due to moisture exposure, creating resistive layers that impede lithium-ion transport. This increased interfacial resistance leads to capacity fade and power density reduction during cycling. Studies have shown that even trace amounts of LiOH (below 1%) can increase the activation energy for ion conduction by up to 15%, severely limiting battery performance at lower temperatures.
LiCl impurities present a different challenge, as they can migrate under electric fields and accumulate at electrode interfaces. This migration creates concentration gradients within the electrolyte, leading to non-uniform current distribution and localized heating. In extreme cases, such hotspots can trigger thermal runaway events, posing significant safety risks, especially in high-energy-density applications.
Residual solvents from synthesis processes, including acetonitrile and tetrahydrofuran, remain a persistent issue in chloride SSEs. These organic compounds can decompose at operating voltages, generating gaseous products that create internal pressure and mechanical stress. Pressure buildup exceeding 2 MPa has been documented in cells with just 0.5% residual solvent content, potentially leading to cell swelling or rupture.
The safety implications extend beyond mechanical failures. Impurities can lower the electrochemical stability window of the electrolyte, catalyzing parasitic reactions at both cathode and anode interfaces. These reactions not only consume active lithium but can also generate flammable byproducts, compromising the inherent safety advantages of solid-state technology.
Performance degradation manifests through multiple mechanisms. Impurities at grain boundaries restrict ion transport pathways, effectively reducing the usable cross-sectional area for conduction. This effect is particularly pronounced in polycrystalline systems, where a 3% impurity concentration can reduce ionic conductivity by up to 40% compared to high-purity samples.
Mitigation strategies must address both synthesis and handling protocols. Advanced purification techniques, including zone refining and solvent extraction, have demonstrated the ability to reduce impurity levels below 100 ppm. Equally important are moisture-controlled processing environments, as post-synthesis exposure to ambient conditions for just 30 minutes can introduce sufficient moisture to form detectable LiOH concentrations.
Characterization Techniques for Impurity Detection and Quantification
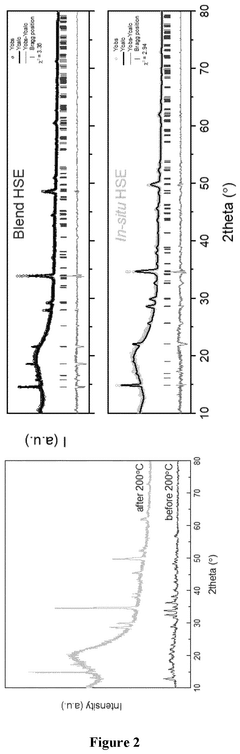
The detection and quantification of impurities in chloride solid-state electrolytes (SSEs) require sophisticated analytical techniques that can identify trace amounts of contaminants with high precision. X-ray diffraction (XRD) serves as a fundamental technique for identifying crystalline impurities such as LiCl and LiOH, providing information about phase composition and structural changes. The characteristic diffraction patterns of these impurities can be distinguished from the host material, allowing for qualitative identification even at relatively low concentrations.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly 7Li and 1H NMR, offers valuable insights into the chemical environment of lithium ions and hydrogen-containing impurities. This technique is especially powerful for detecting LiOH and residual solvents, as it can distinguish between different lithium-containing species based on their chemical shifts and relaxation times. Solid-state NMR has become increasingly important for analyzing SSEs directly in their operational state.
Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy provide complementary information about molecular vibrations, making them excellent tools for identifying organic residual solvents and hydroxide impurities. The OH stretching vibration in LiOH, for instance, produces a characteristic band that can be readily identified and quantified using these techniques.
For more precise quantification, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Optical Emission Spectroscopy (ICP-OES) offer detection limits in the parts-per-billion range for elemental analysis, particularly useful for quantifying chloride and lithium-based impurities. These techniques require sample dissolution but provide exceptional sensitivity for trace impurity detection.
Thermal analysis methods, including Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC), help identify volatile impurities such as residual solvents by monitoring mass changes and heat flow during controlled heating. The characteristic decomposition or evaporation temperatures of different impurities allow for their identification and approximate quantification.
Advanced surface characterization techniques like X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) provide detailed information about surface impurities, which often play a critical role in interfacial resistance and electrochemical stability of SSEs. These techniques can detect impurities at extremely low concentrations and provide spatial distribution information.
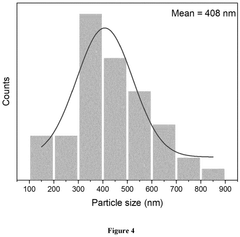
Recent developments in cryo-electron microscopy and atom probe tomography have enabled three-dimensional visualization of impurity distribution within SSEs at near-atomic resolution, offering unprecedented insights into how impurities segregate and affect local structure and ion transport pathways.
Nuclear Magnetic Resonance (NMR) spectroscopy, particularly 7Li and 1H NMR, offers valuable insights into the chemical environment of lithium ions and hydrogen-containing impurities. This technique is especially powerful for detecting LiOH and residual solvents, as it can distinguish between different lithium-containing species based on their chemical shifts and relaxation times. Solid-state NMR has become increasingly important for analyzing SSEs directly in their operational state.
Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy provide complementary information about molecular vibrations, making them excellent tools for identifying organic residual solvents and hydroxide impurities. The OH stretching vibration in LiOH, for instance, produces a characteristic band that can be readily identified and quantified using these techniques.
For more precise quantification, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Optical Emission Spectroscopy (ICP-OES) offer detection limits in the parts-per-billion range for elemental analysis, particularly useful for quantifying chloride and lithium-based impurities. These techniques require sample dissolution but provide exceptional sensitivity for trace impurity detection.
Thermal analysis methods, including Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC), help identify volatile impurities such as residual solvents by monitoring mass changes and heat flow during controlled heating. The characteristic decomposition or evaporation temperatures of different impurities allow for their identification and approximate quantification.
Advanced surface characterization techniques like X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) provide detailed information about surface impurities, which often play a critical role in interfacial resistance and electrochemical stability of SSEs. These techniques can detect impurities at extremely low concentrations and provide spatial distribution information.
Recent developments in cryo-electron microscopy and atom probe tomography have enabled three-dimensional visualization of impurity distribution within SSEs at near-atomic resolution, offering unprecedented insights into how impurities segregate and affect local structure and ion transport pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!