Chloride Solid Electrolytes For All-Solid-State Lithium Batteries: Status And Outlook
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride Solid Electrolytes Background and Objectives
Solid-state lithium batteries represent a significant advancement in energy storage technology, offering potential solutions to the safety, energy density, and longevity limitations of conventional lithium-ion batteries with liquid electrolytes. Among various solid electrolyte candidates, chloride-based solid electrolytes have emerged as promising materials due to their high ionic conductivity, favorable electrochemical stability, and potential for cost-effective manufacturing.
The evolution of chloride solid electrolytes can be traced back to the early 2000s when researchers began exploring alternatives to oxide and sulfide-based systems. Initial chloride electrolytes suffered from low ionic conductivity and poor stability, limiting their practical applications. However, the field experienced a breakthrough in 2018 with the discovery of lithium-rich anti-perovskite chlorides, demonstrating room-temperature ionic conductivities approaching those of liquid electrolytes.
Recent years have witnessed accelerated development in chloride solid electrolytes, with significant improvements in synthesis methods, compositional optimization, and interface engineering. The incorporation of dopants and the development of composite structures have further enhanced their performance characteristics, making them increasingly viable for commercial applications.
The global push toward electrification and renewable energy integration has intensified the demand for advanced battery technologies, positioning all-solid-state batteries as a critical component of future energy systems. Chloride electrolytes, with their unique combination of properties, are positioned to play a pivotal role in this transition.
The primary technical objectives for chloride solid electrolytes research include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability against lithium metal anodes, improving mechanical properties for better processability, and developing scalable manufacturing techniques compatible with existing production infrastructure.
Additionally, research aims to address the moisture sensitivity of chloride electrolytes, which presents challenges for handling and long-term stability. Understanding and mitigating interfacial reactions between chloride electrolytes and electrode materials remains another critical objective, as these interfaces often determine overall battery performance and longevity.
The development trajectory suggests that chloride solid electrolytes could enable the commercialization of all-solid-state lithium batteries with energy densities exceeding 400 Wh/kg, representing a significant improvement over current lithium-ion technologies. This advancement would have profound implications for electric vehicles, grid storage, and portable electronics markets.
The evolution of chloride solid electrolytes can be traced back to the early 2000s when researchers began exploring alternatives to oxide and sulfide-based systems. Initial chloride electrolytes suffered from low ionic conductivity and poor stability, limiting their practical applications. However, the field experienced a breakthrough in 2018 with the discovery of lithium-rich anti-perovskite chlorides, demonstrating room-temperature ionic conductivities approaching those of liquid electrolytes.
Recent years have witnessed accelerated development in chloride solid electrolytes, with significant improvements in synthesis methods, compositional optimization, and interface engineering. The incorporation of dopants and the development of composite structures have further enhanced their performance characteristics, making them increasingly viable for commercial applications.
The global push toward electrification and renewable energy integration has intensified the demand for advanced battery technologies, positioning all-solid-state batteries as a critical component of future energy systems. Chloride electrolytes, with their unique combination of properties, are positioned to play a pivotal role in this transition.
The primary technical objectives for chloride solid electrolytes research include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability against lithium metal anodes, improving mechanical properties for better processability, and developing scalable manufacturing techniques compatible with existing production infrastructure.
Additionally, research aims to address the moisture sensitivity of chloride electrolytes, which presents challenges for handling and long-term stability. Understanding and mitigating interfacial reactions between chloride electrolytes and electrode materials remains another critical objective, as these interfaces often determine overall battery performance and longevity.
The development trajectory suggests that chloride solid electrolytes could enable the commercialization of all-solid-state lithium batteries with energy densities exceeding 400 Wh/kg, representing a significant improvement over current lithium-ion technologies. This advancement would have profound implications for electric vehicles, grid storage, and portable electronics markets.
Market Analysis for All-Solid-State Lithium Batteries
The global market for all-solid-state lithium batteries (ASSLBs) is experiencing significant growth, driven by increasing demand for safer and higher energy density power solutions across multiple sectors. Current market valuations place the ASSLB market at approximately $500 million in 2023, with projections indicating potential growth to reach $2-3 billion by 2030, representing a compound annual growth rate (CAGR) of over 25%.
Electric vehicles (EVs) constitute the primary market driver, accounting for nearly 60% of the projected demand. Major automotive manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery technology, with Toyota alone committing over $13.5 billion toward battery development including solid-state technologies. The automotive sector's push toward electrification creates a particularly strong demand case for chloride-based solid electrolytes due to their potential for enabling higher energy density and faster charging capabilities.
Consumer electronics represents the second largest application segment, currently comprising about 25% of the market. Manufacturers are seeking batteries with improved safety profiles and higher energy densities to enable slimmer device designs and longer operating times between charges. Several major electronics companies have established partnerships with solid electrolyte developers specifically focused on chloride-based systems.
The energy storage sector is emerging as another significant market, particularly for grid-scale applications where safety concerns are paramount. This segment is expected to grow at a CAGR of approximately 30% through 2030, faster than the overall market average.
Regionally, Asia Pacific dominates the market landscape with approximately 65% share, led by Japan, South Korea, and China. These countries have established robust supply chains and significant R&D investments in solid-state battery technologies. North America follows with roughly 20% market share, while Europe accounts for about 15%.
Key market challenges include high production costs, with current manufacturing expenses for chloride solid electrolyte batteries estimated at 3-5 times higher than conventional lithium-ion batteries. Scaling production processes while maintaining electrolyte purity and performance consistency remains a significant hurdle.
Consumer adoption barriers include concerns about long-term reliability, limited performance data in real-world conditions, and uncertainty regarding cycle life. Market analysts suggest that achieving price parity with conventional lithium-ion batteries will be critical for mass market adoption, with projections indicating this could occur between 2028-2030 if current development trajectories continue.
Electric vehicles (EVs) constitute the primary market driver, accounting for nearly 60% of the projected demand. Major automotive manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery technology, with Toyota alone committing over $13.5 billion toward battery development including solid-state technologies. The automotive sector's push toward electrification creates a particularly strong demand case for chloride-based solid electrolytes due to their potential for enabling higher energy density and faster charging capabilities.
Consumer electronics represents the second largest application segment, currently comprising about 25% of the market. Manufacturers are seeking batteries with improved safety profiles and higher energy densities to enable slimmer device designs and longer operating times between charges. Several major electronics companies have established partnerships with solid electrolyte developers specifically focused on chloride-based systems.
The energy storage sector is emerging as another significant market, particularly for grid-scale applications where safety concerns are paramount. This segment is expected to grow at a CAGR of approximately 30% through 2030, faster than the overall market average.
Regionally, Asia Pacific dominates the market landscape with approximately 65% share, led by Japan, South Korea, and China. These countries have established robust supply chains and significant R&D investments in solid-state battery technologies. North America follows with roughly 20% market share, while Europe accounts for about 15%.
Key market challenges include high production costs, with current manufacturing expenses for chloride solid electrolyte batteries estimated at 3-5 times higher than conventional lithium-ion batteries. Scaling production processes while maintaining electrolyte purity and performance consistency remains a significant hurdle.
Consumer adoption barriers include concerns about long-term reliability, limited performance data in real-world conditions, and uncertainty regarding cycle life. Market analysts suggest that achieving price parity with conventional lithium-ion batteries will be critical for mass market adoption, with projections indicating this could occur between 2028-2030 if current development trajectories continue.
Current Status and Challenges of Chloride Solid Electrolytes
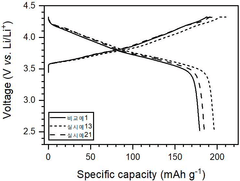
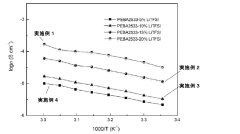
Chloride solid electrolytes have emerged as promising candidates for all-solid-state lithium batteries due to their high ionic conductivity and favorable electrochemical properties. Currently, several chloride-based systems have demonstrated room temperature ionic conductivities exceeding 10^-3 S/cm, comparable to liquid electrolytes. Notable examples include Li3YCl6, Li2ZrCl6, and Li3InCl6, which have attracted significant research attention in recent years.
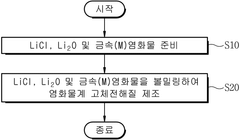
The synthesis of chloride solid electrolytes typically involves mechanochemical methods such as ball milling or solution-based approaches. However, these processes often result in materials with varying degrees of crystallinity and purity, affecting their electrochemical performance. The challenge of achieving consistent, high-quality synthesis remains a significant hurdle for commercial applications.
A major technical limitation of chloride solid electrolytes is their high sensitivity to moisture and air. Most chloride-based systems rapidly degrade upon exposure to ambient conditions, necessitating stringent handling protocols and specialized manufacturing environments. This sensitivity significantly complicates both research efforts and potential industrial scale-up.
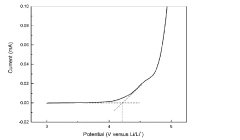
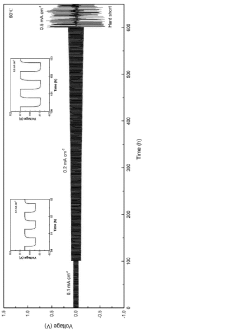
Interface stability represents another critical challenge. While chloride electrolytes generally exhibit better compatibility with lithium metal anodes compared to oxide and sulfide counterparts, they still face issues related to interfacial resistance growth during cycling. The formation of interphases at the electrode-electrolyte interfaces can lead to capacity fading and reduced cycle life.
Mechanical properties of chloride solid electrolytes present additional complications. Many chloride systems exhibit insufficient mechanical strength to withstand the volume changes of electrode materials during cycling. This can lead to contact loss and increased internal resistance, ultimately degrading battery performance over time.
From a global perspective, research on chloride solid electrolytes is primarily concentrated in East Asia (particularly Japan, China, and South Korea), North America, and Europe. Japanese institutions have pioneered many fundamental discoveries, while Chinese research groups have made significant advances in material optimization and cell integration. North American and European efforts have focused on understanding fundamental mechanisms and developing novel synthesis approaches.
The scalability of chloride electrolyte production remains largely unexplored, with most current research conducted at laboratory scales. The transition to industrial-scale manufacturing will require addressing challenges related to batch consistency, processing costs, and environmental considerations associated with chloride-containing materials.
The synthesis of chloride solid electrolytes typically involves mechanochemical methods such as ball milling or solution-based approaches. However, these processes often result in materials with varying degrees of crystallinity and purity, affecting their electrochemical performance. The challenge of achieving consistent, high-quality synthesis remains a significant hurdle for commercial applications.
A major technical limitation of chloride solid electrolytes is their high sensitivity to moisture and air. Most chloride-based systems rapidly degrade upon exposure to ambient conditions, necessitating stringent handling protocols and specialized manufacturing environments. This sensitivity significantly complicates both research efforts and potential industrial scale-up.
Interface stability represents another critical challenge. While chloride electrolytes generally exhibit better compatibility with lithium metal anodes compared to oxide and sulfide counterparts, they still face issues related to interfacial resistance growth during cycling. The formation of interphases at the electrode-electrolyte interfaces can lead to capacity fading and reduced cycle life.
Mechanical properties of chloride solid electrolytes present additional complications. Many chloride systems exhibit insufficient mechanical strength to withstand the volume changes of electrode materials during cycling. This can lead to contact loss and increased internal resistance, ultimately degrading battery performance over time.
From a global perspective, research on chloride solid electrolytes is primarily concentrated in East Asia (particularly Japan, China, and South Korea), North America, and Europe. Japanese institutions have pioneered many fundamental discoveries, while Chinese research groups have made significant advances in material optimization and cell integration. North American and European efforts have focused on understanding fundamental mechanisms and developing novel synthesis approaches.
The scalability of chloride electrolyte production remains largely unexplored, with most current research conducted at laboratory scales. The transition to industrial-scale manufacturing will require addressing challenges related to batch consistency, processing costs, and environmental considerations associated with chloride-containing materials.
Current Technical Solutions for Chloride Solid Electrolytes
01 Lithium chloride-based solid electrolytes
Lithium chloride-based solid electrolytes are widely used in battery applications due to their high ionic conductivity and stability. These electrolytes typically incorporate lithium chloride with other compounds to enhance performance characteristics such as conductivity and electrochemical stability. The formulations often include additives that improve the interface between the electrolyte and electrodes, reducing resistance and enhancing overall battery efficiency.- Lithium chloride-based solid electrolytes: Lithium chloride-based solid electrolytes are widely used in battery applications due to their high ionic conductivity and stability. These electrolytes typically incorporate lithium chloride with other compounds to enhance performance characteristics such as conductivity and electrochemical stability. The formulations may include various additives to improve mechanical properties and interface compatibility with electrodes, making them suitable for next-generation solid-state batteries.
- Halide-based solid electrolyte compositions: Halide-based solid electrolytes, particularly those containing chloride ions, offer advantages in terms of ionic conductivity and electrochemical stability. These compositions often combine multiple metal chlorides to achieve desired properties. The electrolytes can be synthesized through various methods including solid-state reactions, mechanochemical processing, and solution-based approaches. These materials are being developed as alternatives to conventional liquid electrolytes to enhance safety and energy density in battery systems.
- Polymer-chloride composite electrolytes: Polymer-chloride composite electrolytes combine the flexibility and processability of polymers with the ionic conductivity of chloride compounds. These hybrid materials typically consist of a polymer matrix (such as polyethylene oxide or polyvinylidene fluoride) infused with chloride salts. The polymer component provides mechanical stability while the chloride salts facilitate ion transport. These composites can be manufactured as thin films or membranes, making them suitable for various electrochemical devices including batteries and sensors.
- Chloride-based solid electrolytes for high-temperature applications: Specialized chloride-based solid electrolytes designed for high-temperature applications offer thermal stability and maintained conductivity at elevated temperatures. These materials often incorporate refractory components or stabilizing agents to prevent degradation under extreme conditions. Applications include high-temperature fuel cells, sensors, and industrial electrochemical processes. The compositions are engineered to minimize thermal expansion mismatch and maintain structural integrity during thermal cycling.
- Novel synthesis methods for chloride solid electrolytes: Innovative synthesis approaches for chloride solid electrolytes focus on controlling microstructure, grain boundaries, and phase purity to enhance ionic conductivity. These methods include mechanochemical processing, solution-based techniques, and advanced sintering protocols. Some approaches incorporate nanoscale engineering to create materials with optimized ion transport pathways. These synthesis innovations aim to address challenges related to processability, scalability, and performance consistency in chloride-based solid electrolytes.
02 Argyrodite-type chloride solid electrolytes
Argyrodite-type chloride solid electrolytes represent an important class of materials for solid-state batteries. These sulfide-based electrolytes containing chloride ions exhibit superior ionic conductivity at room temperature. The partial substitution of sulfur with chlorine in the crystal structure enhances the lithium ion transport pathways, resulting in improved conductivity. These materials are particularly promising for next-generation energy storage applications due to their stability and performance characteristics.Expand Specific Solutions03 Polymer-chloride composite solid electrolytes
Polymer-chloride composite solid electrolytes combine the flexibility and processability of polymers with the high ionic conductivity of chloride salts. These composites typically consist of a polymer matrix such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF) infused with chloride-containing salts. The polymer component provides mechanical stability while the chloride salts facilitate ion transport. These materials offer advantages in terms of flexibility, ease of manufacturing, and reduced interfacial resistance in battery applications.Expand Specific Solutions04 Metal chloride solid electrolytes for high-temperature applications
Metal chloride solid electrolytes designed for high-temperature applications utilize various metal chlorides such as silver chloride, copper chloride, or alkaline earth metal chlorides. These electrolytes exhibit enhanced ionic conductivity at elevated temperatures, making them suitable for high-temperature batteries, fuel cells, and sensors. The composition typically includes stabilizing agents to prevent degradation and maintain performance under extreme operating conditions. These materials are particularly valuable in industrial settings where high-temperature operation is required.Expand Specific Solutions05 Chloride-doped ceramic solid electrolytes
Chloride-doped ceramic solid electrolytes incorporate chloride ions into ceramic structures to enhance ionic conductivity and electrochemical performance. Common ceramic hosts include oxide-based materials such as NASICON-type structures, perovskites, or garnet-type oxides. The introduction of chloride ions into these ceramic frameworks creates additional defects and ion transport pathways, resulting in improved conductivity. These materials combine the mechanical and thermal stability of ceramics with the enhanced ionic transport properties provided by chloride doping, making them suitable for various electrochemical applications.Expand Specific Solutions
Key Industry Players in Solid-State Battery Development
The chloride solid electrolyte market for all-solid-state lithium batteries is currently in an early growth phase, with significant R&D activity but limited commercialization. Market size remains modest but is projected to expand rapidly as the technology matures. Leading Asian companies including LG Energy Solution, Samsung SDI, and NGK Insulators are at the forefront of development, with academic institutions like Xi'an Jiaotong University and Zhejiang University providing crucial research support. Japanese firms such as Nippon Electric Glass and Maxell are advancing materials engineering, while Korean players like KETI are focusing on manufacturing scalability. The technology is approaching commercial viability, with companies like Hyzon Motors and Nissan-Renault exploring integration into next-generation energy storage systems, though challenges in stability and conductivity remain.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced chloride solid electrolytes for all-solid-state lithium batteries focusing on argyrodite-type Li6PS5Cl materials. Their proprietary synthesis method involves mechanochemical ball milling followed by heat treatment to optimize crystallinity and ionic conductivity. The company has achieved room temperature ionic conductivities exceeding 5 mS/cm with their chloride solid electrolytes. LG's approach includes surface modification of the electrolyte particles with lithium-containing compounds to improve the interfacial stability with cathode materials. They've also developed composite electrolytes by incorporating chloride-based solid electrolytes with oxide materials to enhance mechanical properties while maintaining high ionic conductivity. Their technology includes specialized coating processes to minimize interfacial resistance between the electrolyte and electrodes.
Strengths: High ionic conductivity at room temperature; improved interfacial stability with electrodes; scalable manufacturing processes aligned with existing battery production infrastructure. Weaknesses: Chloride solid electrolytes remain sensitive to moisture requiring strict manufacturing controls; mechanical stability issues during cycling that can lead to contact loss between components.
The Regents of the University of California
Technical Solution: The University of California research teams have developed innovative approaches to chloride solid electrolytes focusing on fundamental understanding and novel material design. Their work has pioneered the development of mixed-anion chloride-based solid electrolytes that incorporate multiple halides (Cl, Br, I) to tune electrochemical properties. UC researchers have established advanced characterization techniques including operando neutron diffraction and synchrotron X-ray studies to elucidate the lithium transport mechanisms in chloride solid electrolytes at the atomic scale. Their technology includes novel synthesis routes using mechanochemical activation followed by controlled crystallization to achieve optimized microstructures. The UC approach has yielded chloride electrolytes with room temperature conductivities reaching 5-7 mS/cm while demonstrating enhanced stability against lithium metal anodes. Their research has also developed computational screening methods to predict new chloride-based compositions with potentially superior properties, leading to several patent-pending formulations.
Strengths: Cutting-edge fundamental research providing deep insights into ion transport mechanisms; innovative mixed-anion approaches offering tunable properties; strong intellectual property portfolio. Weaknesses: Laboratory-scale processes that may face challenges in scaling to industrial production; some compositions require expensive or rare elements that could limit commercial viability.
Critical Patents and Research on Chloride Electrolyte Materials
Chloride-based solid electrolyte, all-solid batteries and manufacturing method thereof
PatentActiveKR1020240000017A
Innovation
- A chloride-based solid electrolyte with a specific composition (Li2+aMCl6-bOc) is produced using ball milling in a controlled environment, incorporating chloride ions (Cl-) to substitute for oxygen ions (O2-), enhancing lithium ion conductivity and electrochemical stability.
Solid electrolyte for all-solid lithium ion battery, manufacturing method thereof, and all-solid lithium ion battery
PatentPendingJP2023152063A
Innovation
- A solid electrolyte comprising a polyether block amide copolymer and a lithium salt is developed, which includes a polyether part and a polyamide part in its structural unit, enhancing battery capacity and cycle stability.
Manufacturing Scalability and Cost Analysis
The scalability of manufacturing processes for chloride solid electrolytes represents a critical challenge in the commercialization of all-solid-state lithium batteries (ASSLBs). Current laboratory-scale synthesis methods, including mechanochemical ball milling and solution-based approaches, face significant barriers when transitioning to industrial production volumes. Ball milling processes, while effective for small batches, demonstrate poor energy efficiency and limited throughput when scaled up, resulting in increased production costs and potential material quality inconsistencies.
Solution-based synthesis methods offer better scalability prospects but introduce additional complexities related to solvent handling, recovery, and environmental considerations. The high sensitivity of chloride solid electrolytes to moisture necessitates stringent atmospheric controls throughout the manufacturing process, requiring specialized equipment and controlled environments that substantially increase production costs.
Raw material costs present another significant barrier to commercial viability. High-purity lithium chloride and other precursor materials command premium prices, with limited suppliers in the global market. Current estimates suggest that material costs alone contribute approximately 40-60% of the total production expenses for chloride solid electrolytes, significantly higher than conventional liquid electrolyte systems.
Energy consumption during processing represents another cost driver, particularly for high-temperature annealing steps required to achieve optimal ionic conductivity in chloride electrolytes. These thermal treatments typically operate at 350-600°C for extended periods, resulting in substantial energy requirements that impact both production costs and environmental footprint.
Quality control processes add further complexity and cost to manufacturing operations. The performance of chloride solid electrolytes is highly sensitive to impurities, crystallinity, and interfacial properties, necessitating sophisticated analytical techniques for in-line monitoring and batch validation. These quality assurance measures, while essential, contribute significantly to overall production expenses.
Recent techno-economic analyses suggest that current manufacturing approaches result in chloride solid electrolyte costs exceeding $1000/kg, approximately 20-30 times higher than conventional liquid electrolytes on a volume-equivalent basis. Industry projections indicate that costs must decrease by at least an order of magnitude to achieve commercial viability in consumer electronics and automotive applications.
Several promising approaches are emerging to address these manufacturing challenges, including continuous processing methods, solvent-free synthesis routes, and automated production systems with integrated quality control. These innovations, coupled with increasing production volumes and supply chain maturation, could potentially reduce costs to the $100-200/kg range within the next 5-7 years, bringing chloride solid electrolytes closer to commercial feasibility for mass-market applications.
Solution-based synthesis methods offer better scalability prospects but introduce additional complexities related to solvent handling, recovery, and environmental considerations. The high sensitivity of chloride solid electrolytes to moisture necessitates stringent atmospheric controls throughout the manufacturing process, requiring specialized equipment and controlled environments that substantially increase production costs.
Raw material costs present another significant barrier to commercial viability. High-purity lithium chloride and other precursor materials command premium prices, with limited suppliers in the global market. Current estimates suggest that material costs alone contribute approximately 40-60% of the total production expenses for chloride solid electrolytes, significantly higher than conventional liquid electrolyte systems.
Energy consumption during processing represents another cost driver, particularly for high-temperature annealing steps required to achieve optimal ionic conductivity in chloride electrolytes. These thermal treatments typically operate at 350-600°C for extended periods, resulting in substantial energy requirements that impact both production costs and environmental footprint.
Quality control processes add further complexity and cost to manufacturing operations. The performance of chloride solid electrolytes is highly sensitive to impurities, crystallinity, and interfacial properties, necessitating sophisticated analytical techniques for in-line monitoring and batch validation. These quality assurance measures, while essential, contribute significantly to overall production expenses.
Recent techno-economic analyses suggest that current manufacturing approaches result in chloride solid electrolyte costs exceeding $1000/kg, approximately 20-30 times higher than conventional liquid electrolytes on a volume-equivalent basis. Industry projections indicate that costs must decrease by at least an order of magnitude to achieve commercial viability in consumer electronics and automotive applications.
Several promising approaches are emerging to address these manufacturing challenges, including continuous processing methods, solvent-free synthesis routes, and automated production systems with integrated quality control. These innovations, coupled with increasing production volumes and supply chain maturation, could potentially reduce costs to the $100-200/kg range within the next 5-7 years, bringing chloride solid electrolytes closer to commercial feasibility for mass-market applications.
Safety and Environmental Considerations
Safety considerations are paramount in the development and implementation of chloride solid electrolytes for all-solid-state lithium batteries. Unlike conventional liquid electrolyte systems, chloride solid electrolytes significantly reduce fire and explosion risks associated with volatile organic solvents. This inherent safety advantage represents one of the primary driving forces behind research into all-solid-state battery technologies. However, several safety concerns specific to chloride-based systems require careful consideration.
The reactivity of chloride solid electrolytes with lithium metal anodes presents a notable safety challenge. Interface reactions can lead to the formation of interphases that may compromise battery performance and potentially create safety hazards during long-term operation. Additionally, some chloride-based materials exhibit sensitivity to moisture and air, which necessitates stringent manufacturing controls and appropriate encapsulation technologies to prevent degradation and potential safety issues.
From an environmental perspective, chloride solid electrolytes offer several advantages over conventional battery technologies. The elimination of toxic and flammable organic solvents contributes to a reduced environmental footprint. Furthermore, many chloride-based materials utilize more abundant elements compared to other solid electrolyte systems, potentially alleviating resource constraints associated with critical materials like cobalt and nickel.
The recyclability of all-solid-state batteries incorporating chloride electrolytes represents another important environmental consideration. Current research indicates that solid-state battery architectures may facilitate more efficient end-of-life recovery of valuable materials compared to conventional lithium-ion batteries. However, specialized recycling processes for chloride-based systems are still under development, and the establishment of effective recycling infrastructure remains a challenge.
Manufacturing processes for chloride solid electrolytes must address potential environmental impacts associated with material synthesis and processing. Some chloride compounds require careful handling due to their hygroscopic nature or potential toxicity. Sustainable manufacturing approaches, including green synthesis methods and reduced energy consumption during production, are being explored to minimize environmental impacts throughout the battery lifecycle.
Regulatory frameworks governing the safety assessment and environmental impact of chloride solid electrolytes continue to evolve. As these materials transition from laboratory research to commercial applications, comprehensive safety testing protocols and environmental impact assessments will be essential to ensure compliance with increasingly stringent regulations and to build consumer confidence in this emerging battery technology.
The reactivity of chloride solid electrolytes with lithium metal anodes presents a notable safety challenge. Interface reactions can lead to the formation of interphases that may compromise battery performance and potentially create safety hazards during long-term operation. Additionally, some chloride-based materials exhibit sensitivity to moisture and air, which necessitates stringent manufacturing controls and appropriate encapsulation technologies to prevent degradation and potential safety issues.
From an environmental perspective, chloride solid electrolytes offer several advantages over conventional battery technologies. The elimination of toxic and flammable organic solvents contributes to a reduced environmental footprint. Furthermore, many chloride-based materials utilize more abundant elements compared to other solid electrolyte systems, potentially alleviating resource constraints associated with critical materials like cobalt and nickel.
The recyclability of all-solid-state batteries incorporating chloride electrolytes represents another important environmental consideration. Current research indicates that solid-state battery architectures may facilitate more efficient end-of-life recovery of valuable materials compared to conventional lithium-ion batteries. However, specialized recycling processes for chloride-based systems are still under development, and the establishment of effective recycling infrastructure remains a challenge.
Manufacturing processes for chloride solid electrolytes must address potential environmental impacts associated with material synthesis and processing. Some chloride compounds require careful handling due to their hygroscopic nature or potential toxicity. Sustainable manufacturing approaches, including green synthesis methods and reduced energy consumption during production, are being explored to minimize environmental impacts throughout the battery lifecycle.
Regulatory frameworks governing the safety assessment and environmental impact of chloride solid electrolytes continue to evolve. As these materials transition from laboratory research to commercial applications, comprehensive safety testing protocols and environmental impact assessments will be essential to ensure compliance with increasingly stringent regulations and to build consumer confidence in this emerging battery technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!