Cathode Compatibility: High-Voltage Layered Oxides With Chloride SSEs
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Voltage Cathode and Chloride SSE Integration Background
The integration of high-voltage layered oxide cathodes with chloride solid-state electrolytes (SSEs) represents a critical frontier in the development of next-generation solid-state batteries (SSBs). This technological pairing has emerged as a promising approach to overcome the energy density limitations of conventional lithium-ion batteries while addressing safety concerns associated with liquid electrolytes.
Historically, the evolution of cathode materials has progressed from lithium cobalt oxide (LCO) to more advanced layered structures such as NMC (nickel-manganese-cobalt) and NCA (nickel-cobalt-aluminum) compositions. These high-voltage cathodes can operate at potentials exceeding 4.3V vs. Li/Li+, offering theoretical energy densities above 800 Wh/kg, substantially higher than commercial lithium-ion batteries (250-300 Wh/kg).
Concurrently, chloride-based solid electrolytes have gained attention due to their exceptional ionic conductivities, often exceeding 10^-3 S/cm at room temperature. Notable examples include Li3YCl6, Li2ZrCl6, and various lithium-rich anti-perovskites. These materials present a compelling alternative to sulfide and oxide electrolytes, balancing high ionic conductivity with improved processability.
The technological convergence of these two components aims to create SSBs with energy densities exceeding 500 Wh/kg while eliminating flammable liquid electrolytes. This advancement would represent a paradigm shift in energy storage, enabling electric vehicles with significantly extended range and consumer electronics with longer operating times between charges.
However, the interface between high-voltage cathodes and chloride electrolytes presents formidable challenges. The high operating potentials of layered oxide cathodes can trigger decomposition of chloride electrolytes, forming resistive interphases that impede lithium-ion transport. Additionally, transition metal dissolution from cathodes can contaminate the electrolyte, further degrading battery performance.
Recent research has focused on interface engineering strategies, including atomic layer deposition of protective coatings, development of gradient composition cathodes, and introduction of buffer layers to mitigate these interfacial reactions. Computational studies have also provided insights into the fundamental mechanisms of degradation, guiding experimental approaches.
The global push toward electrification has accelerated research in this domain, with significant investments from both public and private sectors. Major automotive manufacturers and battery companies have established dedicated research programs targeting the commercialization of high-voltage solid-state batteries incorporating these materials.
As this technology continues to evolve, understanding the complex interplay between cathode structure, surface chemistry, and electrolyte stability becomes increasingly crucial for realizing the full potential of these advanced energy storage systems.
Historically, the evolution of cathode materials has progressed from lithium cobalt oxide (LCO) to more advanced layered structures such as NMC (nickel-manganese-cobalt) and NCA (nickel-cobalt-aluminum) compositions. These high-voltage cathodes can operate at potentials exceeding 4.3V vs. Li/Li+, offering theoretical energy densities above 800 Wh/kg, substantially higher than commercial lithium-ion batteries (250-300 Wh/kg).
Concurrently, chloride-based solid electrolytes have gained attention due to their exceptional ionic conductivities, often exceeding 10^-3 S/cm at room temperature. Notable examples include Li3YCl6, Li2ZrCl6, and various lithium-rich anti-perovskites. These materials present a compelling alternative to sulfide and oxide electrolytes, balancing high ionic conductivity with improved processability.
The technological convergence of these two components aims to create SSBs with energy densities exceeding 500 Wh/kg while eliminating flammable liquid electrolytes. This advancement would represent a paradigm shift in energy storage, enabling electric vehicles with significantly extended range and consumer electronics with longer operating times between charges.
However, the interface between high-voltage cathodes and chloride electrolytes presents formidable challenges. The high operating potentials of layered oxide cathodes can trigger decomposition of chloride electrolytes, forming resistive interphases that impede lithium-ion transport. Additionally, transition metal dissolution from cathodes can contaminate the electrolyte, further degrading battery performance.
Recent research has focused on interface engineering strategies, including atomic layer deposition of protective coatings, development of gradient composition cathodes, and introduction of buffer layers to mitigate these interfacial reactions. Computational studies have also provided insights into the fundamental mechanisms of degradation, guiding experimental approaches.
The global push toward electrification has accelerated research in this domain, with significant investments from both public and private sectors. Major automotive manufacturers and battery companies have established dedicated research programs targeting the commercialization of high-voltage solid-state batteries incorporating these materials.
As this technology continues to evolve, understanding the complex interplay between cathode structure, surface chemistry, and electrolyte stability becomes increasingly crucial for realizing the full potential of these advanced energy storage systems.
Market Analysis for Advanced Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple sectors. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating a compound annual growth rate of 34.2% through 2030, potentially reaching a market value of $3.4 billion.
The integration of high-voltage layered oxide cathodes with chloride solid-state electrolytes (SSEs) represents a particularly promising segment within this expanding market. This technological pairing addresses critical performance limitations in conventional lithium-ion batteries while offering substantial improvements in energy density, safety, and longevity.
Automotive applications currently dominate market demand, accounting for nearly 60% of projected solid-state battery implementation. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13.5 billion in solid-state battery technology development, with particular focus on high-voltage cathode compatibility solutions. The push toward electric vehicles with greater range and faster charging capabilities is creating strong market pull for these advanced battery technologies.
Consumer electronics represents the second largest market segment, with approximately 25% market share. Manufacturers are seeking batteries with higher energy density and improved safety profiles to enable thinner, lighter devices with longer operational times between charges. The compatibility challenges between high-voltage cathodes and chloride electrolytes are particularly relevant in this space, where thermal management constraints are significant.
Geographically, Asia-Pacific leads market development with 45% of global activity, followed by North America (30%) and Europe (20%). Japan and South Korea have established particularly strong positions in chloride-based solid electrolyte research and commercialization pathways.
Market analysis indicates that the interface stability between high-voltage layered oxide cathodes and chloride SSEs represents a critical value inflection point. Solutions addressing this compatibility challenge could potentially unlock a premium market segment valued at $1.2 billion by 2028, with particularly strong growth in aerospace, military, and high-performance portable applications.
Investment patterns show increasing venture capital interest, with funding rounds for startups focused on cathode-electrolyte interface solutions growing from average investments of $25 million in 2020 to $78 million in 2023. This reflects market recognition of the significant commercial potential for technologies that successfully overcome the compatibility challenges between high-voltage layered oxides and chloride solid-state electrolytes.
The integration of high-voltage layered oxide cathodes with chloride solid-state electrolytes (SSEs) represents a particularly promising segment within this expanding market. This technological pairing addresses critical performance limitations in conventional lithium-ion batteries while offering substantial improvements in energy density, safety, and longevity.
Automotive applications currently dominate market demand, accounting for nearly 60% of projected solid-state battery implementation. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13.5 billion in solid-state battery technology development, with particular focus on high-voltage cathode compatibility solutions. The push toward electric vehicles with greater range and faster charging capabilities is creating strong market pull for these advanced battery technologies.
Consumer electronics represents the second largest market segment, with approximately 25% market share. Manufacturers are seeking batteries with higher energy density and improved safety profiles to enable thinner, lighter devices with longer operational times between charges. The compatibility challenges between high-voltage cathodes and chloride electrolytes are particularly relevant in this space, where thermal management constraints are significant.
Geographically, Asia-Pacific leads market development with 45% of global activity, followed by North America (30%) and Europe (20%). Japan and South Korea have established particularly strong positions in chloride-based solid electrolyte research and commercialization pathways.
Market analysis indicates that the interface stability between high-voltage layered oxide cathodes and chloride SSEs represents a critical value inflection point. Solutions addressing this compatibility challenge could potentially unlock a premium market segment valued at $1.2 billion by 2028, with particularly strong growth in aerospace, military, and high-performance portable applications.
Investment patterns show increasing venture capital interest, with funding rounds for startups focused on cathode-electrolyte interface solutions growing from average investments of $25 million in 2020 to $78 million in 2023. This reflects market recognition of the significant commercial potential for technologies that successfully overcome the compatibility challenges between high-voltage layered oxides and chloride solid-state electrolytes.
Technical Challenges in Cathode-Electrolyte Interfaces
The interface between high-voltage layered oxide cathodes and chloride solid-state electrolytes (SSEs) presents significant technical challenges that impede the development of high-performance all-solid-state batteries. The primary issue stems from the chemical and electrochemical instability at this critical junction. When layered oxides such as NMC (nickel-manganese-cobalt) and NCA (nickel-cobalt-aluminum) materials contact chloride-based SSEs, interfacial reactions occur that form resistive layers, increasing impedance and reducing overall battery performance.
These interfacial reactions are particularly problematic during cycling at high voltages (>4.3V vs. Li/Li+), where transition metal dissolution from the cathode accelerates. The dissolved metal ions can migrate through the electrolyte, potentially causing further degradation and safety concerns. Additionally, chloride anions from the SSE may intercalate into the layered oxide structure, disrupting the crystalline framework and causing capacity fade.
Space charge layer formation represents another significant challenge. The difference in chemical potentials between the cathode and chloride SSE creates an electric field at the interface, leading to ion redistribution and the formation of a depletion layer. This phenomenon increases interfacial resistance and hampers lithium-ion transport across the boundary, ultimately limiting rate capability and power density.
Mechanical compatibility issues further complicate the interface dynamics. During cycling, layered oxide cathodes undergo volume changes that can reach 10% or more. These dimensional fluctuations create mechanical stress at the cathode-electrolyte interface, potentially leading to contact loss, crack formation, and increased impedance. The relatively brittle nature of chloride SSEs exacerbates this problem, as they cannot accommodate these volume changes effectively.
Processing challenges also contribute to interface complications. The high sintering temperatures required for chloride SSE densification can trigger undesired reactions with cathode materials, forming secondary phases that impair electrochemical performance. Moreover, achieving intimate contact between the cathode and electrolyte surfaces remains difficult due to their different surface energies and mechanical properties.
Recent research has identified that the cathode-electrolyte interface degradation accelerates at elevated temperatures, with reaction kinetics approximately doubling with every 10°C increase. This temperature sensitivity poses additional challenges for applications requiring operation across wide temperature ranges. Furthermore, the interface degradation mechanisms appear to be path-dependent, with different cycling protocols and voltage windows resulting in varied degradation patterns and failure modes.
These interfacial reactions are particularly problematic during cycling at high voltages (>4.3V vs. Li/Li+), where transition metal dissolution from the cathode accelerates. The dissolved metal ions can migrate through the electrolyte, potentially causing further degradation and safety concerns. Additionally, chloride anions from the SSE may intercalate into the layered oxide structure, disrupting the crystalline framework and causing capacity fade.
Space charge layer formation represents another significant challenge. The difference in chemical potentials between the cathode and chloride SSE creates an electric field at the interface, leading to ion redistribution and the formation of a depletion layer. This phenomenon increases interfacial resistance and hampers lithium-ion transport across the boundary, ultimately limiting rate capability and power density.
Mechanical compatibility issues further complicate the interface dynamics. During cycling, layered oxide cathodes undergo volume changes that can reach 10% or more. These dimensional fluctuations create mechanical stress at the cathode-electrolyte interface, potentially leading to contact loss, crack formation, and increased impedance. The relatively brittle nature of chloride SSEs exacerbates this problem, as they cannot accommodate these volume changes effectively.
Processing challenges also contribute to interface complications. The high sintering temperatures required for chloride SSE densification can trigger undesired reactions with cathode materials, forming secondary phases that impair electrochemical performance. Moreover, achieving intimate contact between the cathode and electrolyte surfaces remains difficult due to their different surface energies and mechanical properties.
Recent research has identified that the cathode-electrolyte interface degradation accelerates at elevated temperatures, with reaction kinetics approximately doubling with every 10°C increase. This temperature sensitivity poses additional challenges for applications requiring operation across wide temperature ranges. Furthermore, the interface degradation mechanisms appear to be path-dependent, with different cycling protocols and voltage windows resulting in varied degradation patterns and failure modes.
Current Interface Engineering Solutions
01 Compatibility mechanisms between high-voltage layered oxides and chloride SSEs
The interface between high-voltage layered oxide cathodes and chloride-based solid-state electrolytes presents unique compatibility challenges. Research focuses on understanding the chemical and electrochemical interactions at this interface, including ion transport mechanisms and potential side reactions. Various coating strategies and interface engineering approaches have been developed to mitigate degradation and enhance compatibility, ensuring stable cycling performance in solid-state batteries using these material combinations.- Layered oxide cathode materials for high-voltage applications: Layered oxide materials, particularly lithium-rich layered oxides, are used as cathode materials in high-voltage battery applications. These materials offer high energy density and operating voltages above 4.5V. The layered structure facilitates lithium-ion diffusion while maintaining structural stability at high voltages. Various compositions including nickel, manganese, and cobalt-based layered oxides have been developed to optimize performance with chloride solid-state electrolytes.
- Chloride-based solid-state electrolytes for high-voltage batteries: Chloride-based solid-state electrolytes offer advantages for high-voltage battery applications including high ionic conductivity and wide electrochemical stability windows. These electrolytes can be formulated with various metal chlorides to enhance compatibility with layered oxide cathodes. The chloride-based SSEs provide improved safety by eliminating flammable liquid electrolytes while enabling stable operation at high voltages, which is critical for next-generation energy storage systems.
- Interface engineering between layered oxides and chloride electrolytes: Interface engineering strategies are employed to improve compatibility between high-voltage layered oxide cathodes and chloride solid-state electrolytes. These include applying protective coatings, buffer layers, and dopants to minimize interfacial resistance and prevent unwanted side reactions. Surface modification of cathode materials with specific elements or compounds can stabilize the cathode-electrolyte interface, reducing degradation during cycling and enabling higher operating voltages.
- Composite electrode structures for enhanced electrochemical performance: Composite electrode structures combining layered oxides with conductive additives and binders are designed to enhance electrochemical performance with chloride solid-state electrolytes. These structures improve electron transport, mechanical stability, and electrolyte contact within the electrode. By optimizing the composition and microstructure of these composite electrodes, issues such as volume changes during cycling and interfacial resistance can be mitigated, leading to better cycling stability at high voltages.
- Advanced manufacturing techniques for layered oxide-chloride SSE batteries: Advanced manufacturing techniques are developed for the fabrication of batteries combining high-voltage layered oxides with chloride solid-state electrolytes. These include specialized sintering processes, cold/hot pressing methods, and novel deposition techniques to create dense, defect-free interfaces. The manufacturing approaches focus on controlling particle size distribution, layer thickness, and interfacial contact to maximize ionic conductivity while maintaining mechanical integrity during battery operation.
02 Novel chloride-based solid electrolyte compositions for high-voltage applications
Advanced chloride-based solid-state electrolytes have been formulated specifically for compatibility with high-voltage layered oxide cathodes. These electrolytes feature optimized ionic conductivity, wide electrochemical stability windows, and reduced interfacial resistance. Innovations include doped chloride structures, composite formulations, and hierarchical designs that maintain stability against high-voltage cathodes while enabling efficient lithium-ion transport, resulting in improved battery performance and cycle life.Expand Specific Solutions03 Modified high-voltage layered oxide cathode materials
High-voltage layered oxide cathodes have been modified to enhance compatibility with chloride solid-state electrolytes. These modifications include surface treatments, elemental doping, and gradient composition structures that stabilize the cathode-electrolyte interface. By controlling surface chemistry and bulk properties, these engineered cathode materials minimize unwanted side reactions with chloride electrolytes, reduce interfacial resistance, and improve cycling stability at high operating voltages.Expand Specific Solutions04 Interface engineering strategies for oxide-chloride systems
Interface engineering approaches have been developed to address the compatibility challenges between high-voltage layered oxides and chloride solid-state electrolytes. These strategies include artificial interlayers, buffer zones, and gradient interfaces that mitigate chemical and electrochemical degradation. Advanced coating technologies using inorganic compounds, polymers, or hybrid materials create stable interfaces that prevent unwanted reactions while maintaining efficient ion transport, significantly improving the electrochemical performance of these battery systems.Expand Specific Solutions05 Testing and characterization methods for oxide-chloride compatibility
Specialized testing and characterization techniques have been developed to evaluate the compatibility between high-voltage layered oxides and chloride solid-state electrolytes. These methods include in-situ/operando spectroscopy, advanced microscopy, impedance analysis, and accelerated aging protocols that reveal interfacial phenomena and degradation mechanisms. Computational modeling approaches complement experimental techniques by predicting thermodynamic stability, reaction pathways, and interface evolution, enabling rational design of more compatible material combinations for next-generation solid-state batteries.Expand Specific Solutions
Key Industry Players in Solid-State Battery Development
The solid-state battery market focusing on high-voltage layered oxide cathodes with chloride solid-state electrolytes (SSEs) is in an early growth phase, with significant R&D activity but limited commercialization. The global solid-state battery market is projected to reach $8-10 billion by 2030, growing at 30% CAGR. Technical maturity remains moderate, with key players demonstrating different approaches: CATL and Factorial are advancing commercial prototypes, while research institutions like Shanghai Institute of Ceramics and University of Maryland focus on fundamental compatibility challenges. Companies like Solid Energies and Honeycomb Battery are developing innovative material interfaces, while established players including Sanyo, Corning, and Nippon Chemi-Con leverage their materials expertise to address chloride SSE stability issues with high-voltage cathodes.
Fundación CIDETEC
Technical Solution: CIDETEC has developed an innovative approach to addressing the compatibility challenges between high-voltage layered oxide cathodes and chloride solid-state electrolytes. Their technology centers on a hybrid electrolyte system that combines the benefits of chloride SSEs with carefully selected interface modifiers. CIDETEC's solution incorporates a thin (<50nm) lithium phosphorous oxynitride (LiPON) buffer layer deposited on high-voltage cathode materials like LNMO and NMC811 before integration with their proprietary chloride-based electrolyte. This buffer layer prevents direct chemical reactions between the cathode and chloride components while maintaining excellent lithium-ion conductivity. Their chloride SSE formulation features a unique composition with partial substitution of larger halide ions that enhance electrochemical stability at high voltages without sacrificing ionic conductivity. CIDETEC has demonstrated cells operating stably at voltages up to 4.8V vs Li/Li+, with energy densities approaching 350 Wh/kg. Their testing shows minimal interfacial impedance growth over hundreds of cycles, indicating effective suppression of parasitic reactions that typically plague high-voltage solid-state systems.
Strengths: Excellent voltage stability enabling use of high-energy cathode materials; innovative buffer layer approach effectively isolates reactive components; demonstrated scalability of coating processes. Weaknesses: Additional processing steps for buffer layer deposition may increase manufacturing complexity; potential challenges with mechanical integrity during thermal cycling; limited data on long-term aging effects beyond laboratory conditions.
Factorial, Inc.
Technical Solution: Factorial has developed proprietary solid-state electrolyte technology specifically addressing cathode compatibility issues with high-voltage layered oxide materials. Their approach involves a chloride-based solid-state electrolyte (SSE) system with modified interfaces that enable stable operation with high-voltage cathodes like NMC and LNMO. The company's technology incorporates protective coatings on cathode particles using atomic layer deposition to create a buffer zone between the reactive cathode surface and chloride electrolyte. This mitigates the chemical degradation typically observed at the cathode-electrolyte interface. Factorial's solution also includes engineered dopants in their chloride SSE formulation that enhance electrochemical stability at high voltages (>4.5V vs Li/Li+) while maintaining high ionic conductivity (>3 mS/cm at room temperature). Their solid-state cells have demonstrated over 80% capacity retention after 500 cycles with high-voltage cathodes.
Strengths: Superior cycle life with high-voltage cathodes compared to conventional electrolytes; scalable manufacturing process compatible with existing battery production lines; reduced interfacial resistance between cathode and electrolyte. Weaknesses: Higher production costs compared to liquid electrolyte systems; potential challenges with mechanical stability during repeated cycling; limited performance data in extreme temperature conditions.
Critical Patents in High-Voltage Cathode-SSE Compatibility
Conducting Polymer/Inorganic Hybrid Solid-State Electrolytes, Lithium Batteries Containing Same, and Production Processes
PatentPendingUS20230238571A1
Innovation
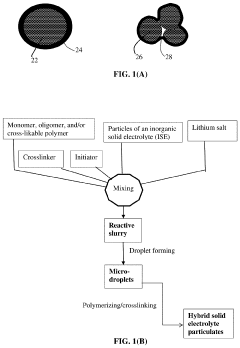
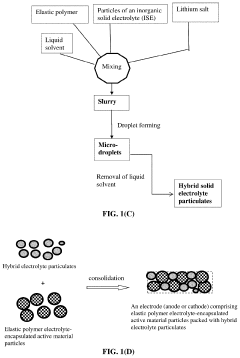
- A hybrid solid electrolyte system comprising inorganic solid electrolyte particles encapsulated in a conducting polymer shell, providing lithium-ion conductivity from 10−6 S/cm to 5×10−2 S/cm, electron conductivity, and a polymer-to-inorganic solid electrolyte ratio from 1/100 to 100/1, allowing for reduced electrolyte volume and improved electrode active material utilization.
High voltage cathode compositions
PatentInactiveEP2277215A1
Innovation
- A cathode composition featuring lithium metal oxide particles coated with a lithium electrode material, such as LiFePO4 or Li4Ti5O12, which are stable at high voltages and maintain capacity, using methods like milling or sol-gel coating to form a layer that reduces interaction with the electrolyte.
Materials Supply Chain Considerations
The global supply chain for high-voltage layered oxide cathodes and chloride solid-state electrolytes (SSEs) presents significant challenges and opportunities for the advancement of solid-state battery technology. Critical raw materials for layered oxide cathodes, including lithium, nickel, manganese, and cobalt, face increasing demand pressure and geopolitical constraints. Particularly, cobalt supply remains concentrated in politically unstable regions, with over 70% of global production originating from the Democratic Republic of Congo, creating vulnerability in the supply chain.
Chloride-based SSEs require different material inputs compared to oxide and sulfide alternatives, potentially offering supply chain diversification. Key elements like lithium, chlorine, and various metals (such as lanthanum, zirconium, or yttrium) used in chloride SSEs have distinct supply dynamics. Notably, rare earth elements used in some chloride SSE formulations face their own supply constraints, with China controlling approximately 85% of global processing capacity.
Manufacturing processes for high-voltage cathodes and chloride SSEs require specialized equipment and expertise, creating potential bottlenecks in scaling production. The synthesis of chloride SSEs demands stringent moisture control, as these materials are typically highly hygroscopic. This necessitates specialized handling facilities and increases production complexity compared to conventional battery materials.
Regional dependencies significantly impact the supply chain resilience. Asia, particularly China, Japan, and South Korea, dominates the cathode materials production landscape. Efforts to establish alternative supply chains in North America and Europe are underway but remain in early stages. The geographical concentration of both raw materials and processing capabilities creates strategic vulnerabilities that must be addressed through diversification strategies.
Recycling and circular economy approaches offer promising pathways to mitigate supply constraints. Recovery of critical materials from end-of-life batteries could reduce primary resource dependencies by up to 30% for certain elements by 2030. However, current recycling technologies are primarily optimized for conventional lithium-ion batteries, requiring adaptation for solid-state configurations incorporating chloride electrolytes.
Emerging alternative material systems, such as manganese-rich cathodes paired with chloride SSEs, may alleviate pressure on constrained supply chains. These alternatives potentially reduce dependency on cobalt and nickel while maintaining compatibility with chloride-based electrolytes. Research into such material systems represents a strategic approach to addressing both technical performance requirements and supply chain considerations simultaneously.
Chloride-based SSEs require different material inputs compared to oxide and sulfide alternatives, potentially offering supply chain diversification. Key elements like lithium, chlorine, and various metals (such as lanthanum, zirconium, or yttrium) used in chloride SSEs have distinct supply dynamics. Notably, rare earth elements used in some chloride SSE formulations face their own supply constraints, with China controlling approximately 85% of global processing capacity.
Manufacturing processes for high-voltage cathodes and chloride SSEs require specialized equipment and expertise, creating potential bottlenecks in scaling production. The synthesis of chloride SSEs demands stringent moisture control, as these materials are typically highly hygroscopic. This necessitates specialized handling facilities and increases production complexity compared to conventional battery materials.
Regional dependencies significantly impact the supply chain resilience. Asia, particularly China, Japan, and South Korea, dominates the cathode materials production landscape. Efforts to establish alternative supply chains in North America and Europe are underway but remain in early stages. The geographical concentration of both raw materials and processing capabilities creates strategic vulnerabilities that must be addressed through diversification strategies.
Recycling and circular economy approaches offer promising pathways to mitigate supply constraints. Recovery of critical materials from end-of-life batteries could reduce primary resource dependencies by up to 30% for certain elements by 2030. However, current recycling technologies are primarily optimized for conventional lithium-ion batteries, requiring adaptation for solid-state configurations incorporating chloride electrolytes.
Emerging alternative material systems, such as manganese-rich cathodes paired with chloride SSEs, may alleviate pressure on constrained supply chains. These alternatives potentially reduce dependency on cobalt and nickel while maintaining compatibility with chloride-based electrolytes. Research into such material systems represents a strategic approach to addressing both technical performance requirements and supply chain considerations simultaneously.
Environmental Impact of Chloride-Based Battery Systems
The environmental implications of chloride-based solid-state electrolyte (SSE) battery systems represent a critical dimension in evaluating their sustainability and long-term viability. When examining high-voltage layered oxide cathodes with chloride SSEs, several environmental considerations emerge throughout the lifecycle of these battery systems.
Raw material extraction for chloride-based SSEs presents a mixed environmental profile compared to conventional lithium-ion batteries. Chloride sources are generally more abundant and require less intensive mining operations than traditional battery materials. However, the extraction of certain metal chlorides still contributes to habitat disruption, water usage concerns, and potential soil contamination in mining regions.
Manufacturing processes for chloride SSE batteries typically require lower processing temperatures than oxide-based alternatives, potentially reducing energy consumption during production. This advantage translates to a smaller carbon footprint during the manufacturing phase, though specialized equipment and controlled environments necessary for chloride handling may partially offset these gains.
The operational phase of chloride-based systems offers significant environmental benefits. Their enhanced thermal stability reduces cooling requirements in battery management systems, decreasing overall energy consumption. Additionally, the extended cycle life of these batteries means fewer replacements and consequently reduced material demand over time.
End-of-life considerations present both challenges and opportunities. Chloride compounds may pose leaching risks if improperly disposed of, potentially contaminating groundwater. However, the recyclability of chloride-based systems appears promising, with simpler separation processes than conventional lithium-ion batteries due to the distinct chemical properties of chloride compounds.
Toxicity profiles of chloride-based systems vary significantly depending on the specific chloride compounds employed. While some metal chlorides present minimal environmental hazards, others may require careful handling and disposal protocols to prevent ecological damage. Comprehensive toxicological assessments remain necessary as these technologies advance toward commercialization.
Water impact deserves particular attention, as certain chloride compounds exhibit high water solubility. This characteristic necessitates robust containment strategies throughout the battery lifecycle to prevent potential contamination of aquatic ecosystems. Conversely, the lower water requirements during manufacturing compared to conventional batteries may represent a net positive in water-stressed regions.
Regulatory frameworks governing chloride-based battery systems continue to evolve, with increasing emphasis on lifecycle assessment and circular economy principles. Future environmental certifications and standards will likely influence the commercial viability and public acceptance of these emerging battery technologies.
Raw material extraction for chloride-based SSEs presents a mixed environmental profile compared to conventional lithium-ion batteries. Chloride sources are generally more abundant and require less intensive mining operations than traditional battery materials. However, the extraction of certain metal chlorides still contributes to habitat disruption, water usage concerns, and potential soil contamination in mining regions.
Manufacturing processes for chloride SSE batteries typically require lower processing temperatures than oxide-based alternatives, potentially reducing energy consumption during production. This advantage translates to a smaller carbon footprint during the manufacturing phase, though specialized equipment and controlled environments necessary for chloride handling may partially offset these gains.
The operational phase of chloride-based systems offers significant environmental benefits. Their enhanced thermal stability reduces cooling requirements in battery management systems, decreasing overall energy consumption. Additionally, the extended cycle life of these batteries means fewer replacements and consequently reduced material demand over time.
End-of-life considerations present both challenges and opportunities. Chloride compounds may pose leaching risks if improperly disposed of, potentially contaminating groundwater. However, the recyclability of chloride-based systems appears promising, with simpler separation processes than conventional lithium-ion batteries due to the distinct chemical properties of chloride compounds.
Toxicity profiles of chloride-based systems vary significantly depending on the specific chloride compounds employed. While some metal chlorides present minimal environmental hazards, others may require careful handling and disposal protocols to prevent ecological damage. Comprehensive toxicological assessments remain necessary as these technologies advance toward commercialization.
Water impact deserves particular attention, as certain chloride compounds exhibit high water solubility. This characteristic necessitates robust containment strategies throughout the battery lifecycle to prevent potential contamination of aquatic ecosystems. Conversely, the lower water requirements during manufacturing compared to conventional batteries may represent a net positive in water-stressed regions.
Regulatory frameworks governing chloride-based battery systems continue to evolve, with increasing emphasis on lifecycle assessment and circular economy principles. Future environmental certifications and standards will likely influence the commercial viability and public acceptance of these emerging battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!