How Proteins Are Attached To PVDF Membrane?
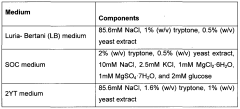
Protein-PVDF Binding Background and Objectives
The attachment of proteins to polyvinylidene fluoride (PVDF) membranes is a critical process in various biochemical and biomedical applications, particularly in protein analysis techniques such as Western blotting. This technology has evolved significantly since its inception in the 1970s, driven by the need for more efficient and reliable protein detection methods.
PVDF membranes have become a preferred substrate for protein binding due to their unique properties, including high protein binding capacity, chemical resistance, and mechanical strength. The hydrophobic nature of PVDF allows for strong interactions with proteins, making it an ideal material for immobilizing a wide range of biomolecules.
The primary objective of protein-PVDF binding is to achieve stable and efficient immobilization of proteins on the membrane surface while maintaining their biological activity and accessibility for subsequent analysis. This process aims to maximize the signal-to-noise ratio in protein detection assays and ensure reproducible results across different experimental conditions.
Over the years, researchers have focused on optimizing the protein-PVDF binding process to address several key challenges. These include improving binding efficiency, reducing non-specific interactions, enhancing protein retention during downstream processing, and minimizing protein denaturation during the binding process.
The evolution of protein-PVDF binding techniques has been closely linked to advancements in membrane manufacturing, surface modification strategies, and protein chemistry. Innovations in these areas have led to the development of various PVDF membrane types with different pore sizes, surface chemistries, and binding capacities, catering to diverse research needs.
Current research in this field is driven by the increasing demand for high-sensitivity protein detection methods in proteomics, diagnostics, and drug discovery. Scientists are exploring novel approaches to enhance protein-PVDF interactions, such as the incorporation of functional groups on the membrane surface, the use of cross-linking agents, and the development of hybrid materials combining PVDF with other polymers or nanoparticles.
The ongoing technological advancements in protein-PVDF binding are expected to contribute significantly to the development of more sensitive and specific protein analysis tools. These improvements will likely have far-reaching implications in various fields, including molecular biology, clinical diagnostics, and personalized medicine, by enabling more accurate and comprehensive protein profiling and characterization.
Market Analysis for Protein Immobilization Technologies
The protein immobilization technology market has witnessed significant growth in recent years, driven by increasing demand in various applications such as diagnostics, drug discovery, and proteomics research. The global market for protein immobilization technologies is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years.
One of the key factors fueling market growth is the rising adoption of protein immobilization techniques in the pharmaceutical and biotechnology industries. These sectors rely heavily on protein-based assays and screening methods for drug development and target identification. The increasing focus on personalized medicine and biomarker discovery has further boosted the demand for efficient protein immobilization technologies.
The healthcare sector represents a major end-user segment for protein immobilization technologies, particularly in the development of diagnostic tools and biosensors. The growing prevalence of chronic diseases and the need for rapid, accurate diagnostic methods have created a substantial market opportunity for protein immobilization solutions.
Academic and research institutions also contribute significantly to the market demand, as protein immobilization techniques are essential for various studies in molecular biology, biochemistry, and related fields. The continuous influx of research funding in life sciences further supports market growth.
Geographically, North America holds the largest share of the protein immobilization technology market, followed by Europe. This dominance can be attributed to the presence of major pharmaceutical and biotechnology companies, well-established research infrastructure, and high R&D investments in these regions. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years, driven by increasing healthcare expenditure, growing research activities, and rising investments in life sciences.
The market for protein immobilization on PVDF membranes, specifically, forms a crucial segment within the broader protein immobilization technology market. PVDF membranes are widely used due to their excellent protein binding capacity, chemical resistance, and compatibility with various detection methods. The demand for PVDF membrane-based protein immobilization solutions is particularly strong in Western blotting applications, a technique extensively used in molecular biology and proteomics research.
Key market players in the protein immobilization technology space are continuously innovating to improve binding efficiency, reduce non-specific interactions, and enhance overall performance. This innovation drive is expected to lead to the development of advanced PVDF membrane products with superior protein attachment capabilities, further stimulating market growth.
Current Challenges in Protein-PVDF Attachment
Despite the widespread use of PVDF membranes in protein blotting applications, several challenges persist in achieving optimal protein attachment. One of the primary issues is the hydrophobic nature of PVDF membranes, which can lead to non-specific binding and high background noise. This hydrophobicity can also result in uneven protein distribution across the membrane surface, affecting the accuracy and reproducibility of results.
Another significant challenge is the variability in protein binding efficiency across different types of proteins. Proteins with varying molecular weights, isoelectric points, and structural characteristics may exhibit different affinities for the PVDF membrane. This inconsistency can lead to difficulties in standardizing protocols and interpreting results, especially when working with complex protein mixtures or comparing different protein samples.
The transfer process itself presents additional challenges. Incomplete transfer of proteins from gels to PVDF membranes can occur, particularly for high molecular weight proteins or those with extreme isoelectric points. This incomplete transfer can result in loss of valuable sample and underrepresentation of certain proteins in the final analysis.
Membrane pore size and uniformity also play crucial roles in protein attachment. While PVDF membranes are available in various pore sizes, selecting the optimal size for a given application can be challenging. Larger pore sizes may allow for better protein transfer but can compromise retention, while smaller pores may impede the transfer of larger proteins.
Post-transfer handling of PVDF membranes poses additional challenges. The membranes are susceptible to drying, which can lead to irreversible protein denaturation and loss of antigenicity. This necessitates careful handling and storage procedures to maintain the integrity of attached proteins.
The choice of blocking agents and washing buffers can also impact protein attachment and subsequent detection. Overly aggressive blocking or washing steps may lead to protein loss, while insufficient blocking can result in high background noise. Striking the right balance is crucial but can be difficult to achieve consistently across different experimental setups.
Lastly, the increasing demand for quantitative analysis in protein blotting applications has highlighted the limitations in achieving precise and reproducible protein quantification on PVDF membranes. Factors such as transfer efficiency, binding capacity, and signal linearity can all affect the accuracy of quantitative measurements, presenting ongoing challenges for researchers seeking to obtain reliable quantitative data from PVDF-based protein blots.
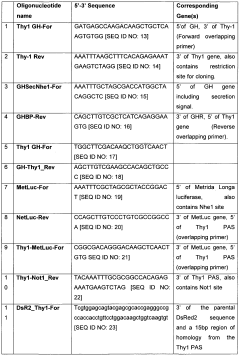
Existing Protein-PVDF Attachment Methods
01 Surface modification of PVDF membranes
Various techniques are used to modify the surface of PVDF membranes to enhance protein attachment. These modifications can include plasma treatment, chemical grafting, or coating with hydrophilic polymers. Such treatments alter the surface properties of the membrane, making it more suitable for protein binding.- Surface modification of PVDF membranes: Various techniques are used to modify the surface of PVDF membranes to enhance protein attachment. These methods include plasma treatment, chemical grafting, and coating with hydrophilic polymers. Such modifications improve the membrane's hydrophilicity and introduce functional groups that facilitate protein binding.
- Protein immobilization techniques: Different approaches are employed to immobilize proteins on PVDF membranes. These include covalent binding, physical adsorption, and affinity-based attachment. The choice of method depends on the specific protein and application requirements, aiming to maintain protein activity and stability.
- Nanostructured PVDF membranes for protein attachment: Nanostructured PVDF membranes are developed to increase surface area and improve protein binding capacity. These membranes may incorporate nanofibers, nanoparticles, or nanoporous structures, enhancing the overall performance in protein attachment applications.
- Composite PVDF membranes for enhanced protein binding: Composite PVDF membranes are created by blending PVDF with other materials or incorporating additives. These composites aim to combine the desirable properties of PVDF with additional features that promote protein attachment, such as increased hydrophilicity or specific binding sites.
- Characterization and optimization of PVDF membranes for protein attachment: Various analytical techniques and optimization methods are used to characterize and improve PVDF membranes for protein attachment. These include surface analysis, protein binding assays, and performance evaluations under different conditions to enhance the efficiency and specificity of protein attachment.
02 Functionalization of PVDF membranes
PVDF membranes can be functionalized with specific chemical groups to improve protein attachment. This process involves introducing reactive groups onto the membrane surface, which can then form covalent bonds with proteins. Common functionalization methods include adding carboxyl, amine, or epoxy groups to the membrane surface.Expand Specific Solutions03 Nanoparticle incorporation in PVDF membranes
Incorporating nanoparticles into PVDF membranes can enhance their protein binding capacity. Nanoparticles such as metal oxides or carbon-based materials can be blended with the PVDF polymer during membrane fabrication. These nanocomposite membranes often exhibit improved surface area and binding sites for proteins.Expand Specific Solutions04 Protein immobilization techniques on PVDF
Various methods are employed to immobilize proteins on PVDF membranes. These can include physical adsorption, covalent binding, or affinity-based attachment. The choice of immobilization technique depends on the specific protein and the intended application, with each method offering different advantages in terms of binding strength and protein activity retention.Expand Specific Solutions05 Characterization of protein attachment on PVDF
Techniques for characterizing protein attachment to PVDF membranes are crucial for optimizing and validating the binding process. These may include spectroscopic methods, microscopy techniques, or protein quantification assays. Such characterization helps in understanding the efficiency of protein attachment and the distribution of proteins on the membrane surface.Expand Specific Solutions
Key Players in Protein Binding and Membrane Technology
The protein attachment to PVDF membranes is a competitive field in the biotechnology sector, currently in a growth phase. The market size is expanding due to increasing applications in proteomics, diagnostics, and drug discovery. Technologically, the process is relatively mature, with ongoing refinements. Companies like Arkema France SA and BASF Plant Science LLC are key players in PVDF membrane production, while academic institutions such as Zhejiang University and Oxford University Innovation Ltd. contribute to research advancements. Pharmaceutical companies like Otsuka Pharmaceutical Co., Ltd. and Baxter International, Inc. are leveraging this technology for various applications. The competition is driven by innovations in membrane properties, protein binding efficiency, and application-specific optimizations.
Arkema France SA
Baxter International, Inc.
Innovative Approaches in Protein-PVDF Binding
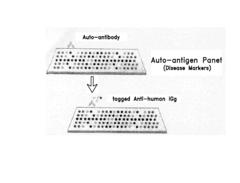
- A substrate comprising a rigid support with a hydrophobic polymeric layer, specifically a PVDF layer, that allows for dry-state protein immobilization with minimal diffusion and low background signal, enabling high-density protein spotting and improved signal-to-noise ratios.
- Genetically modifying eukaryotic cells to inhibit the expression of genes involved in GPI anchor synthesis, specifically targeting PGAP2 and PIG-A, to reduce the shedding of GPI-anchored proteins, thereby enhancing the production of non-GPI modified proteins and simplifying the purification process.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of attaching proteins to PVDF membranes are crucial aspects that require thorough examination. PVDF membranes are widely used in various biomedical applications due to their excellent chemical resistance and mechanical properties. However, when proteins are attached to these membranes, several factors must be carefully evaluated to ensure the safety and efficacy of the resulting biomaterial.
One primary consideration is the potential for immune responses. The attachment of proteins to PVDF membranes can alter the surface properties of the material, potentially triggering unwanted immune reactions in the body. This is particularly important in applications such as implantable devices or biosensors, where long-term biocompatibility is essential. Researchers must carefully select and modify proteins to minimize immunogenicity while maintaining their desired functionality.
The stability of the protein-PVDF interface is another critical factor. The attachment method must ensure that proteins remain securely bound to the membrane under physiological conditions. Unstable attachments could lead to protein detachment, potentially causing adverse effects or compromising the intended function of the biomaterial. Various techniques, such as covalent bonding or affinity-based immobilization, are employed to enhance the stability of protein attachment.
Toxicity concerns also play a significant role in biocompatibility assessments. While PVDF itself is generally considered biocompatible, the introduction of proteins and any chemical linkers or modifiers used in the attachment process may introduce toxic elements. Comprehensive in vitro and in vivo studies are necessary to evaluate the potential cytotoxicity, genotoxicity, and systemic toxicity of the protein-modified PVDF membranes.
The preservation of protein structure and function upon attachment is crucial for both safety and efficacy. Improper attachment methods can lead to protein denaturation or conformational changes, potentially exposing hidden epitopes or altering the protein's biological activity. This not only affects the intended function of the biomaterial but may also introduce unexpected biological interactions or immune responses.
Sterilization compatibility is another important consideration. The protein-PVDF composite must withstand sterilization processes without compromising the integrity of the attached proteins or the membrane itself. Common sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving can potentially damage proteins or alter the surface properties of the PVDF membrane.
Long-term stability and degradation behavior of the protein-PVDF composite must also be carefully evaluated. This includes assessing the potential for protein leaching, membrane degradation, and the formation of potentially harmful degradation products over time. Understanding these aspects is crucial for predicting the long-term performance and safety of the biomaterial in various applications.
Scalability and Cost-Effectiveness Analysis
The scalability and cost-effectiveness of attaching proteins to PVDF membranes are crucial factors in determining the viability of this technique for large-scale applications. The process of protein attachment to PVDF membranes can be scaled up through various methods, each with its own cost implications and efficiency considerations.
One approach to improving scalability is the use of automated systems for membrane handling and protein application. These systems can significantly increase throughput and reduce labor costs, making the process more economically viable for large-scale operations. However, the initial investment in such equipment can be substantial, requiring a careful cost-benefit analysis before implementation.
Another factor affecting scalability is the optimization of protein binding protocols. By fine-tuning parameters such as protein concentration, incubation time, and buffer composition, it is possible to enhance the efficiency of protein attachment while minimizing waste. This optimization can lead to significant cost savings in terms of reagent usage and processing time.
The choice of PVDF membrane itself also plays a role in scalability and cost-effectiveness. Higher-quality membranes may have a higher initial cost but can offer improved protein binding capacity and durability, potentially reducing overall expenses in the long run. Conversely, lower-cost membranes might be more suitable for certain applications where high performance is not critical.
Reagent costs are a significant consideration in the scalability of protein attachment to PVDF membranes. Developing strategies to recycle or reduce the consumption of expensive reagents can greatly improve the economic viability of large-scale operations. This may involve the implementation of closed-loop systems or the exploration of alternative, more cost-effective reagents that maintain the desired protein attachment efficiency.
The energy requirements for the protein attachment process should also be evaluated when considering scalability. Techniques that minimize energy consumption, such as room-temperature binding protocols or energy-efficient incubation systems, can contribute to improved cost-effectiveness, especially in large-scale applications.
Lastly, the potential for process integration and streamlining should be explored. By combining protein attachment with subsequent analytical or purification steps, it may be possible to reduce overall processing time and costs. This integrated approach can enhance the scalability of the entire workflow, making it more attractive for industrial applications.