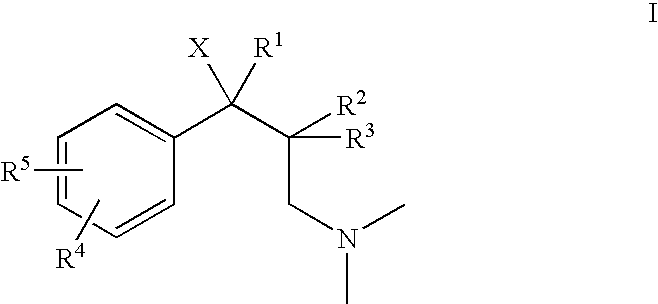
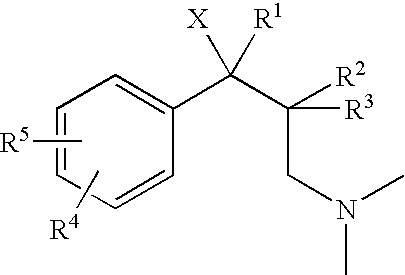
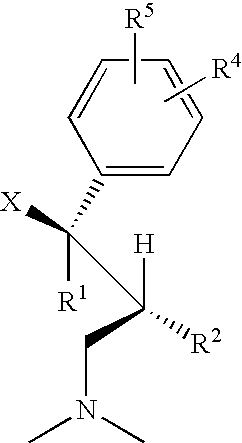
1-phenyl-3-dimethylaminopropane compounds with a pharmacological effects
a technology of phenyl-3dimethylaminopropane and phenyl-3dimethylaminopropane, which is applied in the direction of phosphorous compound active ingredients, drug compositions, group 5/15 element organic compounds, etc., can solve problems such as a series of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045]
(2RS,3RS)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentan-3-ol Hydrochloride(1)
[0046]207.63 g (1.11 mole) 3-bromoanisole dissolved in 400 ml dry tetrahydrofuran were added drop-wise to 26.99 g (1.11 mole) magnesium turnings in 150 ml dry tetrahydrofuran so that the reaction mixture boiled gently. After the addition of 3-bromoanisole was complete the mixture was heated under reflux for one hour and thereafter was cooled to 5-10° C. 128.30 g (0.89 mole) 1-dimethylamino-2-methylpentan-3-one dissolved in 400 ml tetrahydrofuran were added at this temperature. The reaction mixture was allowed to stand overnight and then cooled again to 5-10° C. The Grignard solution was decomposed by the addition of 300 ml of 20% ammonium chloride solution. The reaction mixture was diluted with 400 ml ether, the organic phase was separated off and the aqueous phase was extracted twice with 250 ml ether. The combined organic phases were dried over sodium sulphate. After removing the solvent by dis...
example 2
[0047]
Enantiomers of (1):[0048](−)-(2S,3S)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentan-3-ol Hydrochloride(−1)
and[0049](+)-(2R,3R)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentan-3-ol Hydrochloride(+1).
[0050]The base was released from (1) with dichloromethane / sodium hydroxide solution. After drying the solution dichloromethane was distilled off under vacuum. The racemate was then separated on the chiral HPLC column. The hydrochlorides, which had a melting point of 150-151° C., were prepared from the enantiomers obtained by reaction with trimethylchlorosilane / water in 2-butanone.
(−1):
[0051]yield: 42% theoretical
[0052][α]DRT=−31.8° (c=0.99; methanol)
(+1):
[0053]yield: 41% theoretical
[0054][α]D=RT+33.0° (c=0.96; methanol)
example 3
[0055]
(2RS,3RS)-3-(3,4-dichlorophenyl)-1-dimethylamino-2-methylpentan-3-ol Hydrochloride (2)
[0056]39 g of crude mixture were prepared analogously to Example 1 from 15 g (105 mmol) 1-dimethylamino-2-methylpentan-3-one, 35.5 g (157 mmole) 4-bromo-1,2-dichlorobenzene and 3.8 g (157 mmole) magnesium turnings. This mixture was introduced on to a 7×40 cm column packed with silica gel and eluted with 4:1 ethyl acetate / methanol. 14.9 g of base were obtained, from which 11.2 g of hydrochloride (2) (31% theoretical) with a melting point of 183-184° C. were obtained with trimethylchlorosilane / water in 2-butanone / diisopropyl ether.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com