Immune function modulating agents
a technology of immune function and modulating agent, which is applied in the field of agents, can solve the problems of food allergy development, uninvestigated preventive and/or therapeutic effects of lactobacilli actually taken up by animals, and unfavorable evaluation of therapeutic effects against food allergies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Gastric Acid Resistance Assay and Bile Acid Resistance Assay
[0071]273 lactobacillus strains of the Lactobacillus genus isolated from human feces were subjected to the following assays:
1) Gastric Acid Resistance Assay:
[0072]Lactobacillus bacteria were washed twice with physiological saline, and 1 ml of the lactobacillus suspension solution was added to 9 ml of filter sterilized artificial gastric fluid (NaCl (0.2%) and pepsin (1:5000, Tokyo Chemical Industry) (0.35%)) (pH 2). After aerobic culture at 37° C. for 2 hours, 1 ml was taken from this artificial gastric fluid containing the lactobacilli and added to 9 ml of phosphate buffer (67 mM, pH 6.5) to terminate the reaction. The number of live bacteria before and after contact with the artificial gastric fluid was measured using the Lactobacilli MRS Agar (Difco), and the viability rate (%) and gastric acid resistance (%) were calculated.
2) Bile Acid Resistance Assay:
[0073]10 μl of lactobacilli precultured twice (37° C., 18 hours) in...
example 2
Preparation of Freeze-dried lactobacillus Bacteria
[0077]The 20 lactobacillus strains of the Lactobacillus genus selected in Example 1 were precultured twice (37° C., 18 hours) in Lactobacilli MRS Broth. The precultures were inoculated in the sane medium at 1% and cultured at 37° C. for 18 hours. Bacteria were collected, then washed twice with physiological saline and once with sterile distilled water, sterilized by heating at 75° C. for 60 minutes, and freeze-dried. Freeze-dried bacterial powder was used in the in vitro assays and animal administration experiments below.
example 3
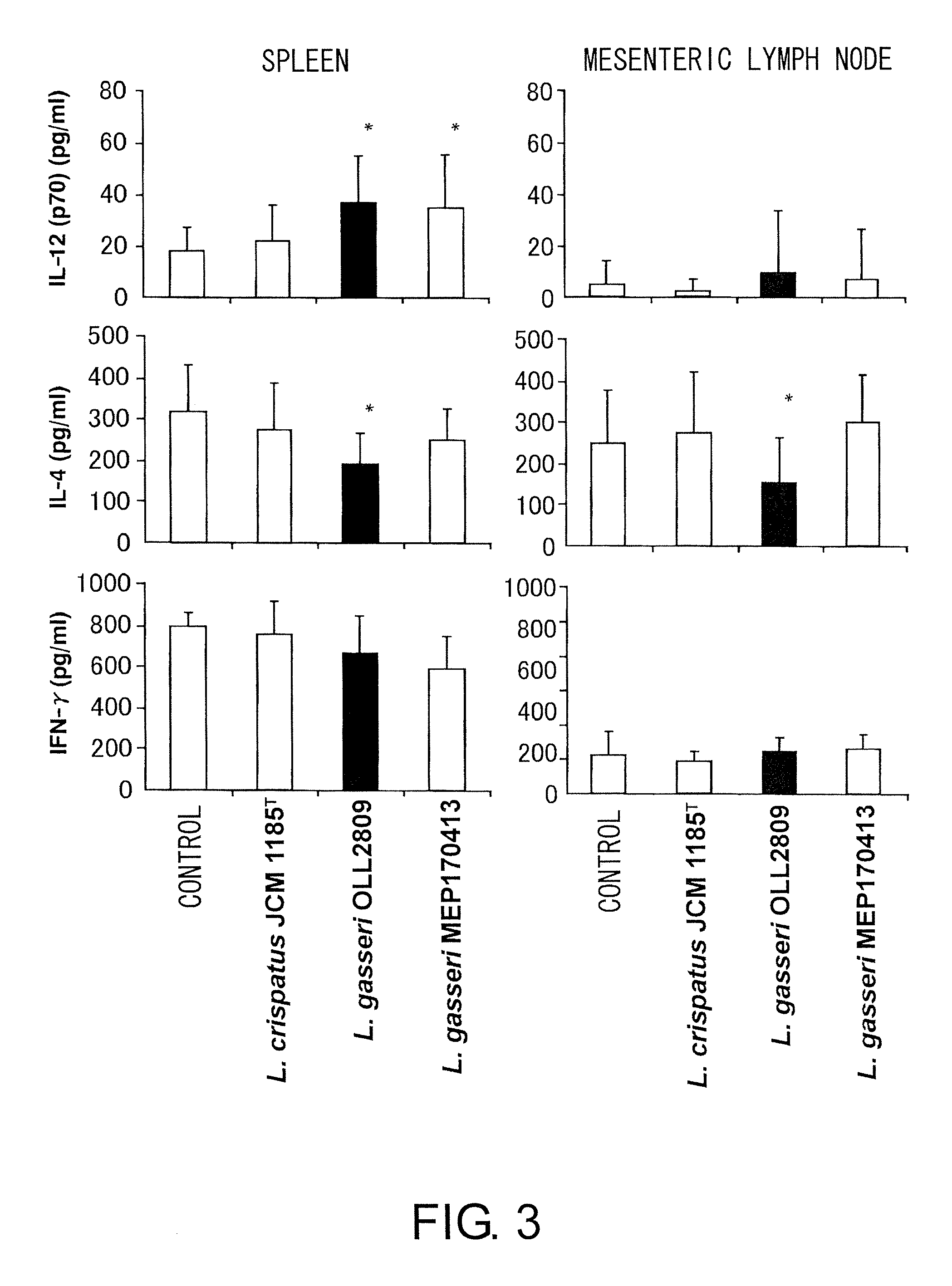
Assays for Evaluating the Effect of Promoting IL-12 Production from Mouse-derived Spleen Cells and the Th1 / Th2 Balance-improving Effect
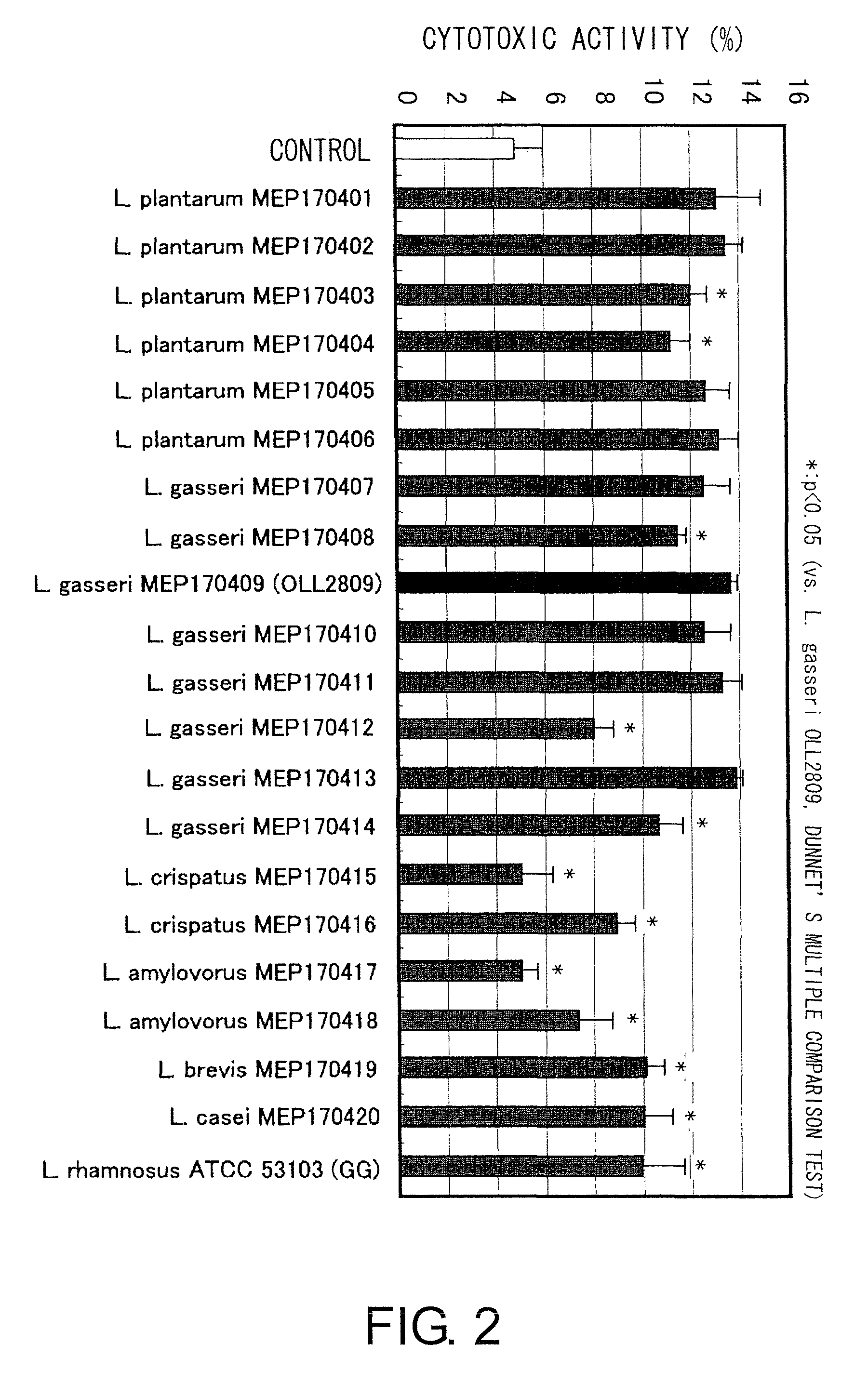
[0078]The gastric acid-resistant and bile acid-resistant 20 lactobacillus strains of the Lactobacillus genus which were selected in Example 1 were evaluated by the methods below.
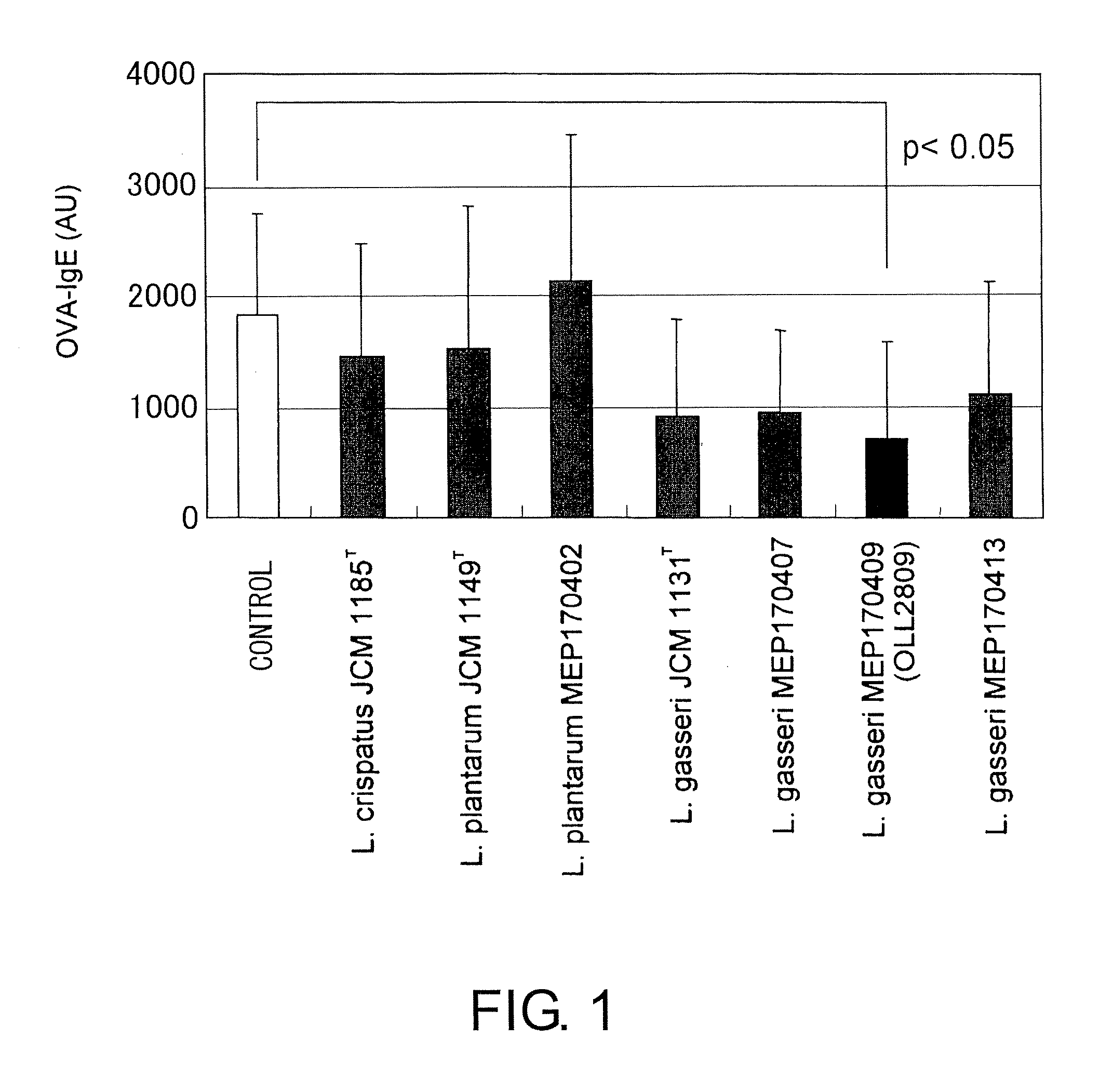
[0079]20 μg of ovalbumin (hereinafter also referred to as OVA, Wako Pure Chemical Industries) and 2 mg of aluminum hydroxide (Wako Pure Chemical Industries) were intraperitoneally administered to six-week-old male BALB / c mice (n=4, Japan SLC). Eight days later, the spleens were removed. Spleen cells from which erythrocytes have been removed were resuspended at 2.5×106 cells / ml in 10% FCS-RPMI 1640 medium (Gibco) containing 1 μg / ml of heat-treated lactobacilli prepared in Example 2 and 100 μg / ml of OVA, and cultured in a 5% CO2 incubator for six days. Type strains of the Lactobacillus genus (L. plantarum JCM 1149T, L. gasseri JCM 1131T, L. crispatus JCM 1185T, L. amylovorus JC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com