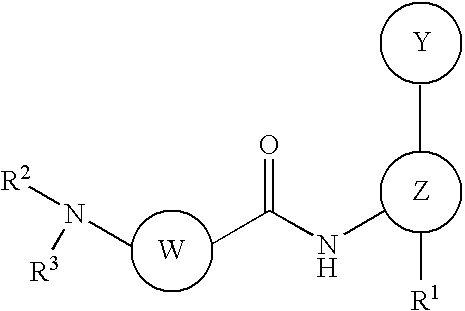
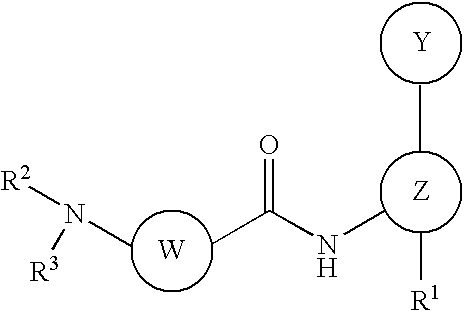
Disubstituted aniline compounds
a technology of aniline compounds and substituted aniline, which is applied in the field of substituted aniline compounds, can solve the problems of excess proliferation of leukemic cell lines and inability to complete differentiation, and achieve the effect of inhibiting the proliferation of such cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0229]
Methyl 4-(dibenzylamino)benzoate
[0230]To a solution of methyl 4-iodobenzoate (1100 mg, 0.38 mmol) in dimethylacetamide (5 mL) is added N-benzyl-1′-phenylmethanamine (221 μL, 1.15 mmol) and K3PO4 (405 mg, 1.9 mmol). The reaction is degassed by freeze-pump-thaw three times. Pd[Pd(t-Bu)3]2 (39 mg, 0.076 mmol) is added and reaction is allowed to stir at 100° C. for 12 hours overnight. The crude reaction mixture was diluted with EtOAc (25 mL) and then washed with dH2O (1×10 mL). The organic layer was dried over MgSO4, filtered, concentrated in vacuo. The crude residue was purified by reverse-phase chromatography (10-100% MeCN / H2O with 0.05% TFA). The appropriate fractions were combined, diluted with EtOAc (20 mL) and washed with sat.'d aq. NaHCO3 (1×10 mL). The organic layer was dried over MgSO4, filtered, and concentrated in vacuo and gave the desired methyl 4-(dibenzylamino)benzoate which was confirmed by MS: cal'd 332.2 (MH+), exp 332.2 (MH+).
example 2
[0231]
N-(2-aminophenyl)-4-(dibenzylamino)benzamide
[0232]To a solution of methyl 4-(dibenzylamino)benzoate (52 mg, 0.16 mmol) in a 2:1 mixture of THF / dH2O was added LiOH (10 mg, 0.48 mmol). Tetra-butyl ammonium hydroxide (2 mL, 0.1N solution in toluene / MeOH) was added to the reaction and allowed to stir at room temperature for 6 hours. The solvent was concentrated in vacuo and the reaction mixture was azeotroped with MeOH several times then placed on the high vacuum for 2 hours to dry to provide the desired 4-(dibenzylamino)benzoic acid which was confirmed by MS: cal'd 318.1 (MH+), exp 318.1 (MH+).
[0233]To a solution of 4-(dibenzylamino)benzoic acid (50 mg, 0.16 mmol) in DMF was added EDC (181 mg, 0.95 mmol), HOBt (128 mg, 0.95 mmol) and phenylenediamine (171 mg, 1.58 mmol) were which was allowed to stir at room temperature for 12 hours. The crude reaction mixture was diluted with EtOAc (20 mL) and then washed with sat.'d aq. NaHCO3 (1×10 mL). The organic layer was dried over MgSO4, ...
example 3
[0234]
Methyl 4-{[2-(dimethylamino) ethyl]amino}benzoate
[0235]Methyl 4-iodobenzoate (1 g, 3.82 mmol), XANTPHOS (0.033 g, 0.057 mmol), Pd2(dba)3 (0.035 g, 0.038 mmol), Cs2CO3 (1.741 g, 5.34 mmol) was added to a sealed tube with septa and purged with argon 3×. N,N-dimethyl-1,2-ethanediamine (0.404 g, 4.58 mmol) and 1,4-Dioxane (4 mL) was then added and was stirred at 110° C. for Overnight. The reaction mixture was cooled to room temp and diluted with ethyl acetate and filtered through celite. The organic solvent was concentrated. The product was used without further purification. MS: cal'd 223 (MH+), exp 223 (MH+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com