Neutralizing antibodies that bind to the zika virus domain iii envelope region
a zika virus and envelope region technology, applied in the field of neutralizing antibodies that bind to the zika virus domain iii envelope region, can solve the problems of cross-reacting antibodies that fail to neutralize, and the spectrum of devastating neurodevelopmental aberrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0143]Serologic Responses to ZIKV in Brazil and Mexico
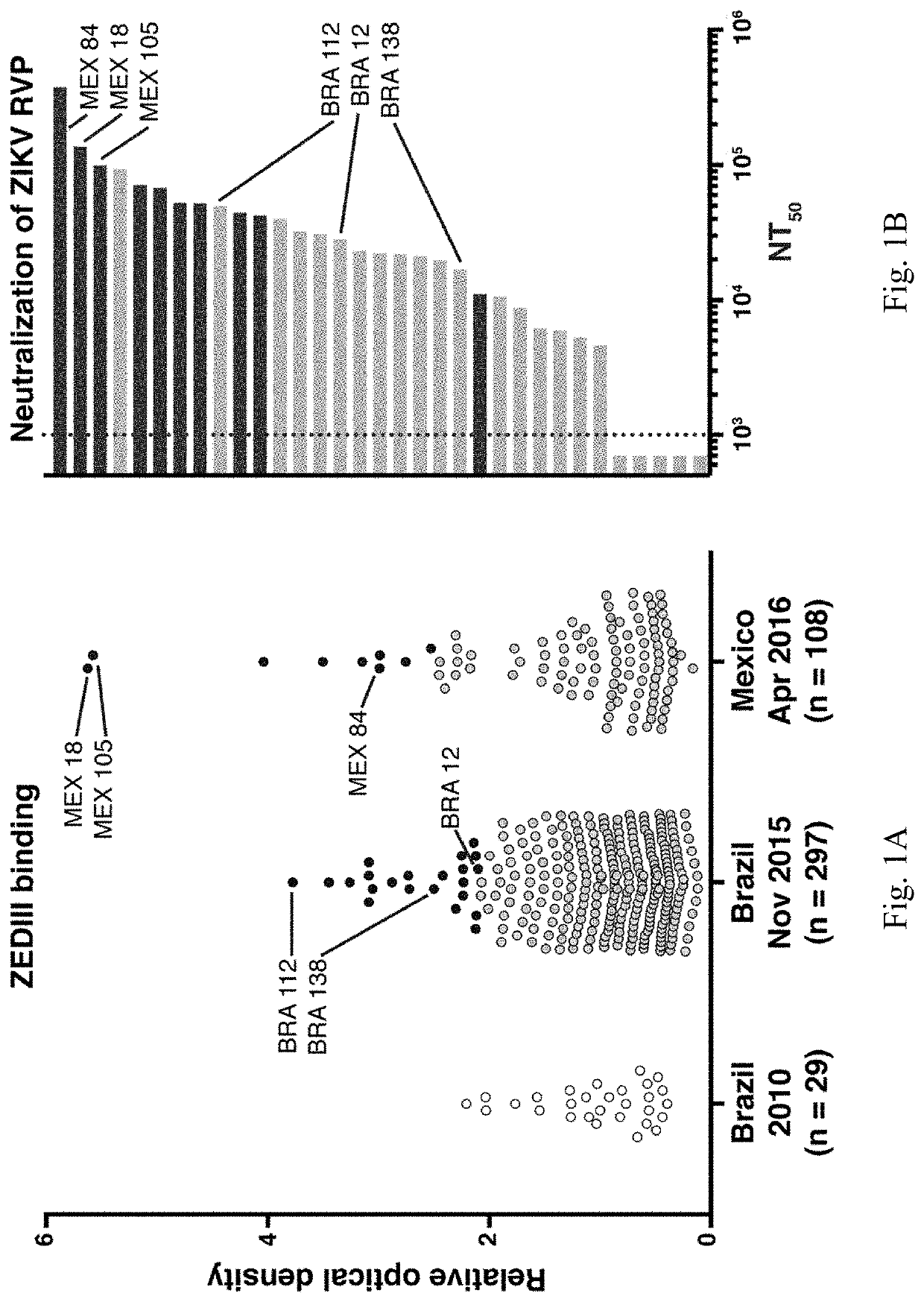
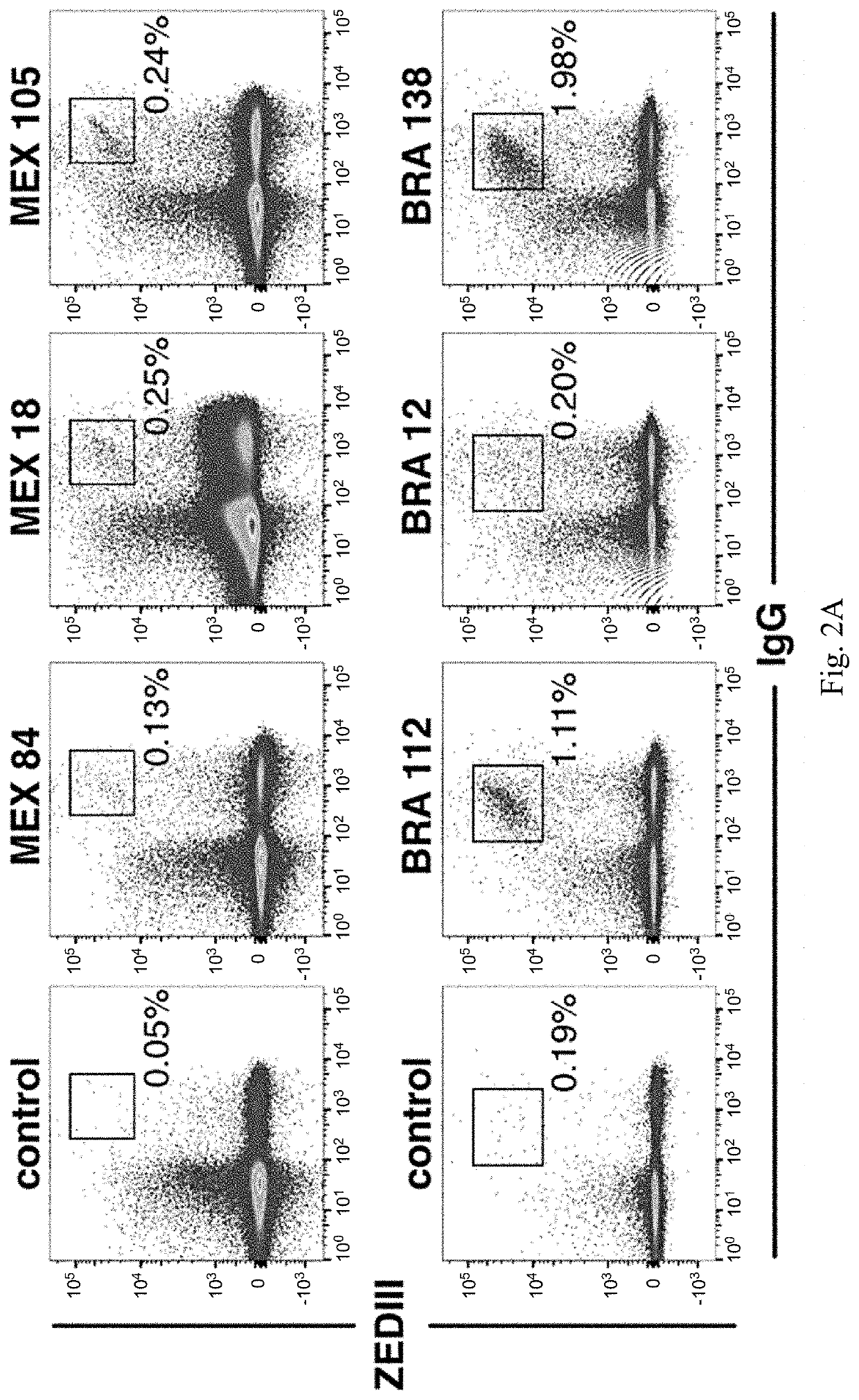
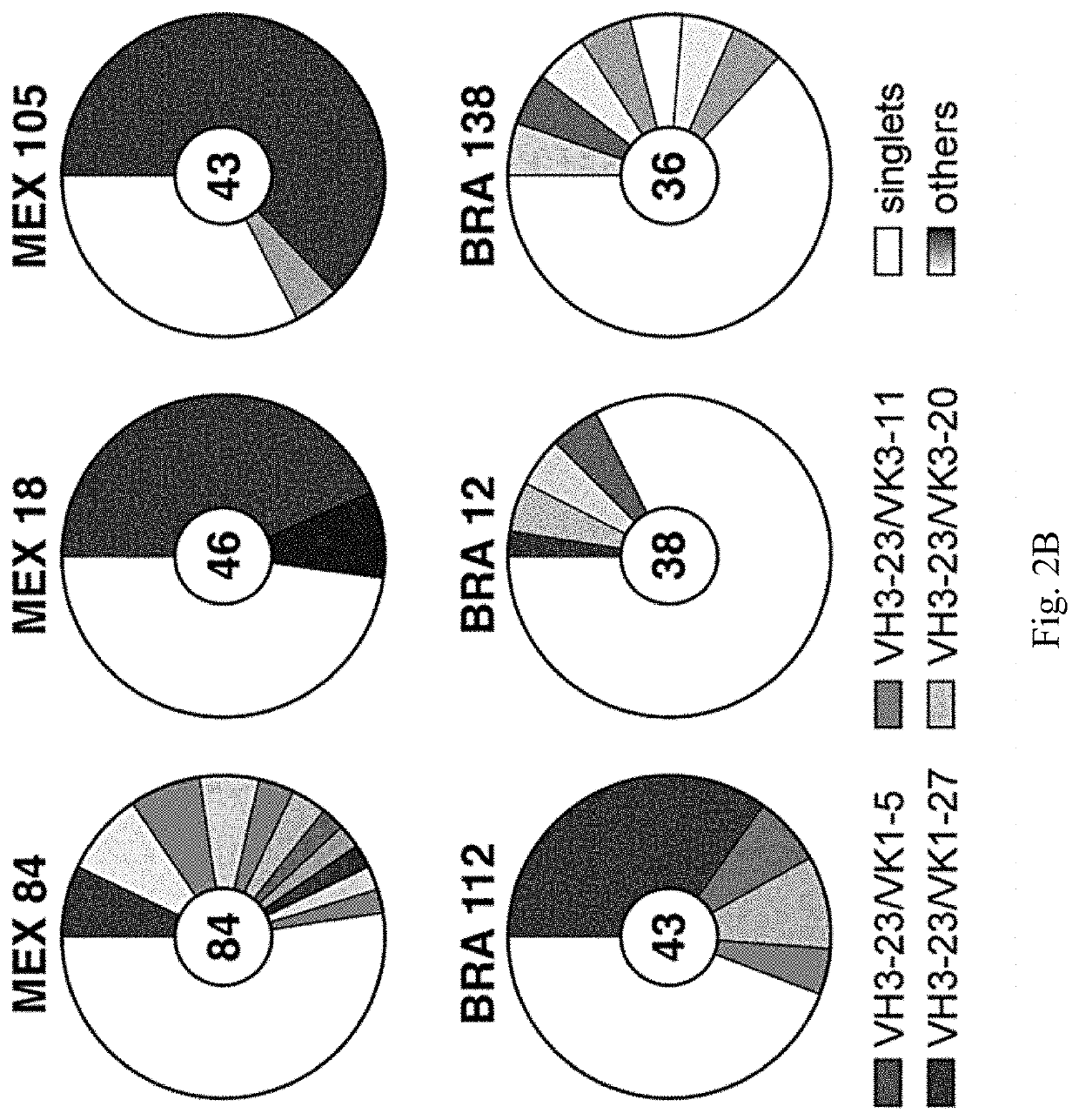
[0144]Individuals infected with pathogens display a spectrum of antibody responses ranging from low levels of non-neutralizing antibodies to high titers of neutralizing antibodies. To determine whether a population infected with ZIKV also displays a range of antibody responses we screened 405 individuals living in ZIKV epidemic areas for serum IgG capable of binding to ZIKV E Domain III (ZEDIII, FIG. 1A).
[0145]Nearly three hundred sera were obtained in November 2015, shortly after ZIKV was introduced in Salvador, Brazil, from participants who were enrolled in a prospective study in 2013 from Pau da Lima, an urban slum community within the city (Cardoso et al., 2015; Felzemburgh et al., 2014; Hagan et al., 2016). An additional 108 sera were from Santa Maria Mixtequilla, a rural town in Oaxaca, Mexico. ZIKV infections were documented by PCR in Santa Maria Mixtequilla at the time of sample collection in April of 2016. Dengue virus (...
example 2
[0166]This Example provides a description of materials and methods used to obtain the results discussed above.
Experimental Model and Subjects Details
[0167]Human Subjects
[0168]Samples of peripheral blood were obtained upon consent from community participants of cohort studies in Pau da Lima (Brazil) and Santa Maria Mixtequilla (Mexico) under protocols approved by the ethical committees of the Rockefeller University (IRB DRO-0898), Yale University (IRB HIC 1603017508), FIOCRUZ (CAAE 63343516.1.0000.5028), Hospital Geral Roberto Santos (1.998.103), and National Institute of Respiratory Diseases (C16-16). Information regarding sex and age of study participants can be obtained upon request. Details on the size of the cohorts and time when samples were obtained is listed in the Results section of the manuscript.
[0169]Mice
[0170]IFNAR1− / − mice were obtained from The Jackson Laboratory and bred and maintained in the AAALAC-certified facility of the Rockefeller University. Mice were specific ...
example 3
[0297]This Example extends the disclosure of Examples 1 and 2 above. Zika virus (ZIKV) infection causes severe neurologic complications and fetal aberrations1. As discussed in Examples 1 and 2, human monoclonal antibody Z004 is a VH3-23 / VK1-5 antibody that recognizes the lateral ridge of the Envelope Domain III (EDIII) of ZIKV, is a potent neutralizer in vitro, and prevents disease in mice. In this Example we demonstrate that when Z004 is administered to macaques for prophylaxis, it leads to emergence of resistant ZIKV variants bearing mutations in the antibody target site. As discussed above, Z021 has a structure in complex with the antigen that reveals a distinct although overlapping epitope on EDIII. Z021 potently neutralizes ZIKV in vitro and prevents disease in mice. This Example shows that in a clinically relevant macaque model, prophylactic co-administration of Z004 with Z021 is protective and suppresses emergence of resistant variants. Thus, a combination of two potent human...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com