Modified pluripotent cells
a technology of pluripotent cells and modified cells, applied in the field of regenerative cell therapy, can solve the problems that rh(d) and rh(c) antigens confer significant risk of hemolytic disease of fetus and newborns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Human iPSCs
[0224]The Human Episomal iPSC Line was derived from CD34+ cord blood (Cat. No. A33124, Thermo Fisher Scientific) using a three-plasmid, seven-factor (SOKMNLT; SOX2, OCT4 (POU5F1), KLF4, MYC, NANOG, LIN28, and SV40L T antigen) EBNA-based episomal system from ThermoFisher. This iPSC line is considered to have a zero footprint as there was no integration into the genome from the reprogramming event. It has been shown to be free of all reprogramming genes. The iPSCs have a normal XX karyotype and endogenous expression of pluripotent markers like OCT4, SOX2, NANOG (as shown by RT-PCR) OCT4, SSEA4, TRA-1-60 and TRA-1-81 (as shown by ICC). In directed differentiation and teratoma analyses, these hiPSCs retained their differentiation potential for the ectodermal, endodermal, and mesodermal lineages. In addition, vascular, endothelial, and cardiac lineages were derived with robust efficiencies.
[0225]Several gene-delivery vehicles for iPSC generation were successfully used, in...
example 2
n of Human HIP Cells
[0229]Hypoimmune pluripotent (HIP) cells were generated as disclosed in WO2018 / 132783 and U.S. Prov. App. Nos. 62 / 698,941, 62 / 698,965, 62 / 698,973, 62 / 698,978, 62 / 698,981, and 62 / 698,984, each of which are incorporated by reference herein in their entirety. Human Cas9 iPSC underwent 2 gene-editing steps. In the first step, CRISPR technology was performed by a combined targeting of the coding sequence of human beta-2-microglobuline (B2M) gene with the CRISPR sequence 5′-CGTGAGTAAACCTGAATCTT-3′ and the coding sequence of human CIITA gene with the CRISPR sequence 5′-GATATTGGCATAAGCCTCCC-3′. Linearized CRISPR sequence with T7 promoter was used to synthesize gRNA as per the kit's instructions (MEGAshortscript T7 Transcription Kit, Thermo Fisher). The collected in-vitro transcription (IVT) gRNA was then purified via the MEGAclear Transcription Clean-Up Kit. For IVT gRNA delivery, singularized cells were electroporated with 300 ng IVT gRNA using a Neon electroporation sy...
example 3
Rejection in Rhesus Macaque Monkeys and Pigs
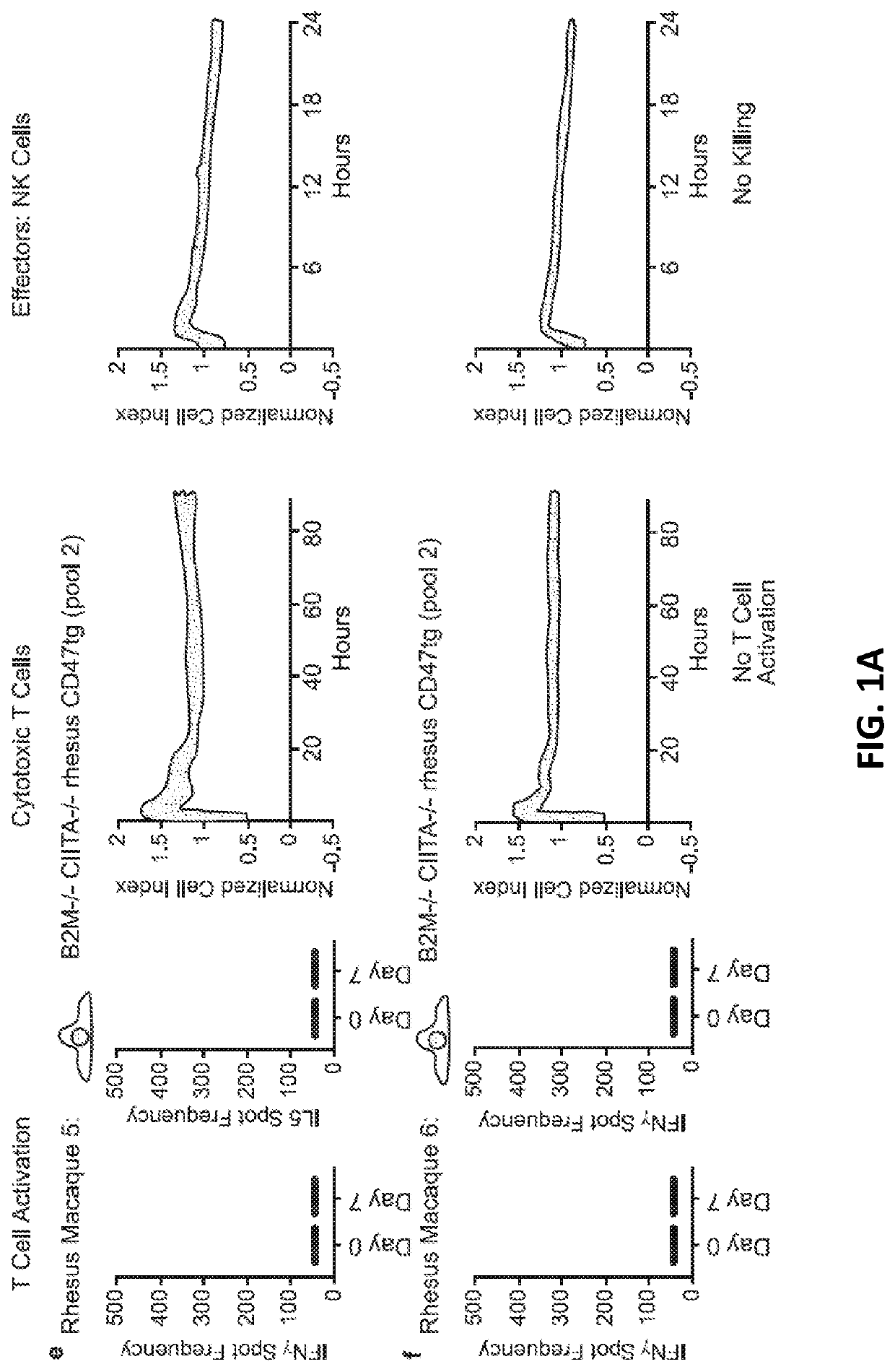
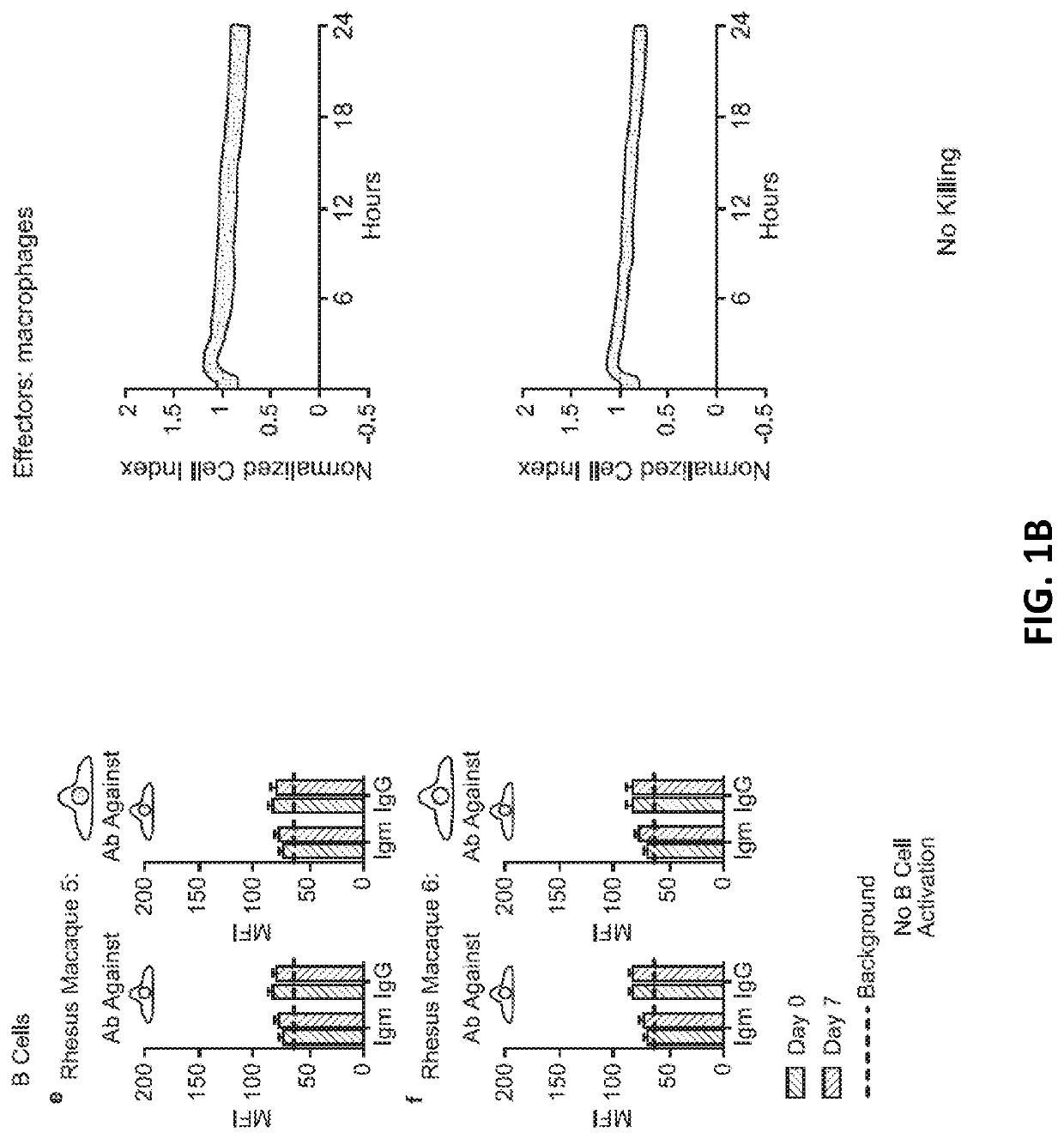
[0231]10 million hypoimmunogenic B2M− / − CIITA− / − rhesus CD47 tg iPSC derived endothelial cells (expressing luciferase) were injected subcutaneously into rhesus macaque monkeys and cells were longitudinally followed using bioluminescence imaging; each animal was injected intravenously with 100 mg / ml D-luciferin (Perkin Elmer, San Jose, Calif.) via a peripheral vessel for in vivo imaging using a Xenogen IVIS® 200 Series imaging system (Caliper Life Sciences, Alameda, Calif., Cat. No. 122799). Our pre-transplant screening assays and validation studies predicted that the cells would not be rejected, resulting in stable BLI signals. The BLI-signals decreased, however, on day 6 and were not detected by day 16. In addition, “bumps” were observed on the injection sites (data not shown). Blood was drawn from the same monkeys and T cell, cytotoxic T cell, NK cell (FIG. 1A), B cell (DSA; donor specific antibodies), or macrophages activation (FIG. 1B)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| Rh | aaaaa | aaaaa |
| β- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com