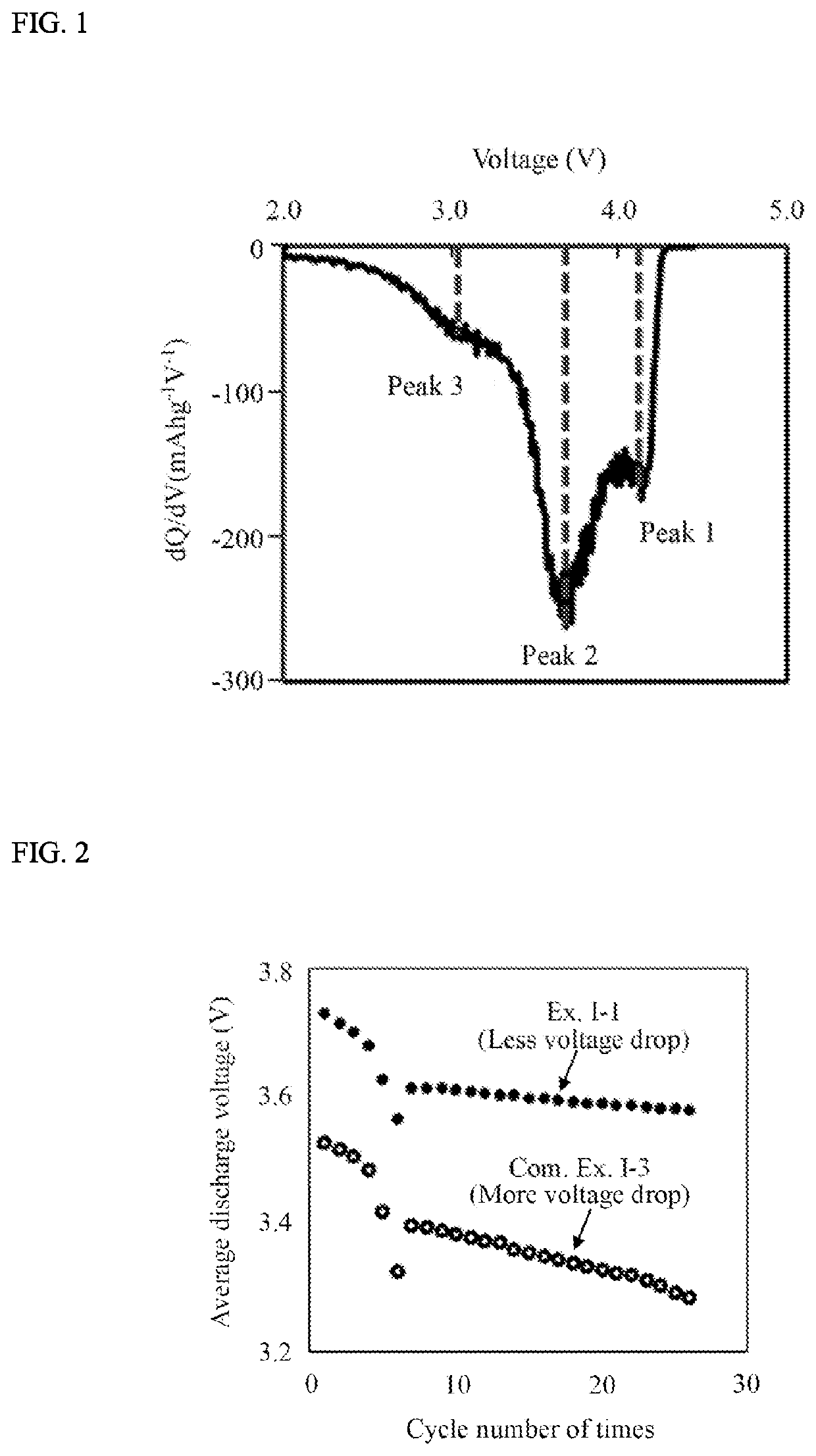
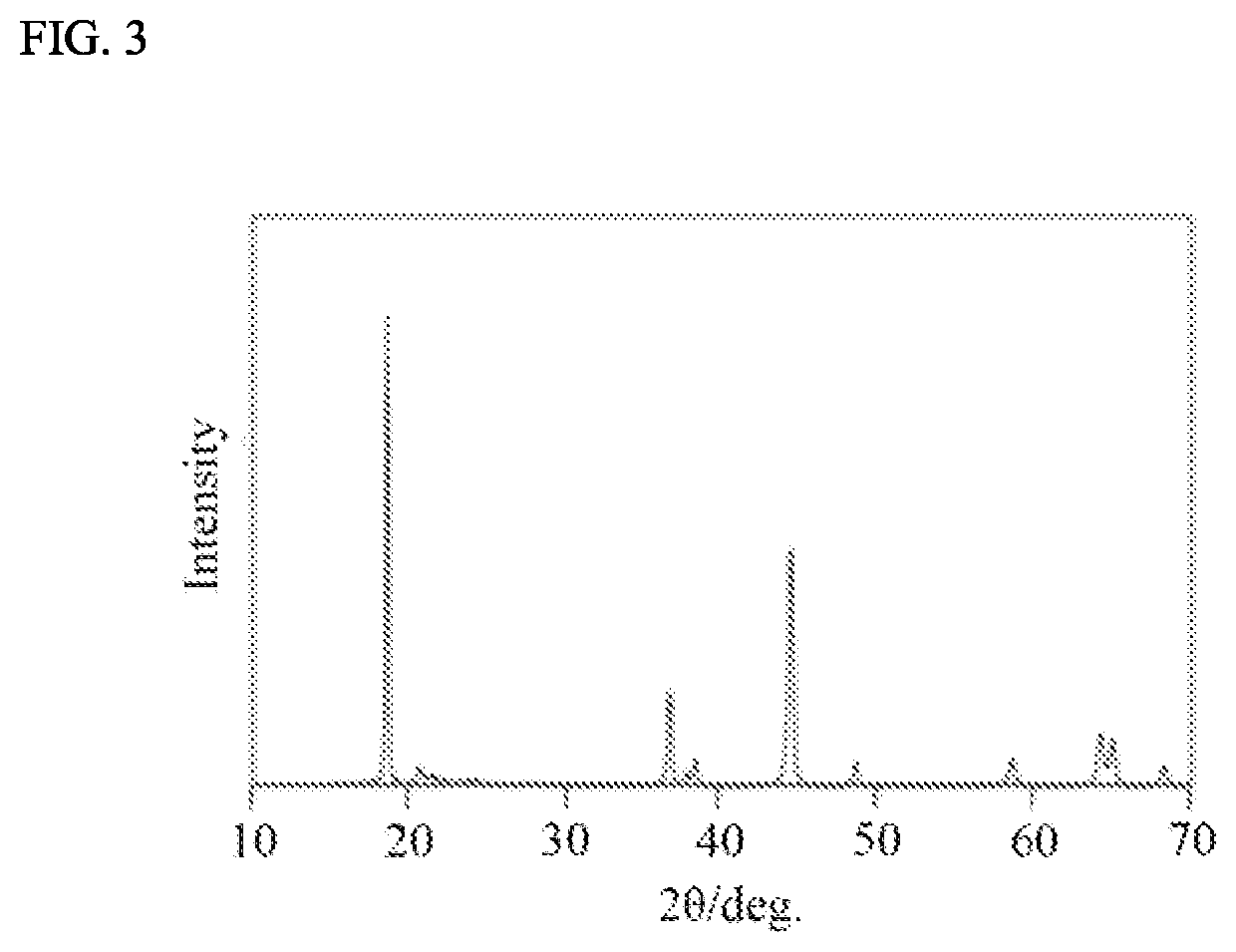
Positive Electrode Active Material And Method For Producing Same, And Non-Aqueous Electrolyte Secondary Battery Using Same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i-1
[0198]Were prepared 0.1 mol / L of a nickel sulfate aqueous solution and 0.1 mol / L of a manganese sulfate aqueous solution. The nickel sulfate aqueous solution and the manganese sulfate aqueous solution were mixed with each other so that a molar ratio of nickel and manganese was adjusted to Ni:Mn=0.35:0.65 to give a mixture solution. Was prepared 1 mol / L of a sodium carbonate aqueous solution. To a sealed type reaction vessel was supplied 8 L of water, and a temperature inside the reaction vessel was kept at 40° C. under nitrogen gas circulation. Drops of the mixture solution and the sodium carbonate aqueous solution were continuously put to the reaction vessel with stirring at a speed of 5 mL / min. Simultaneously drops of the sodium carbonate aqueous solution were put to the reaction vessel so that a reacting solution in the reaction vessel had pH of 8.00 (±0.01). During reaction of the reacting solution, only a filtrate was removed from a reaction system by using a concentration vess...
example i-2
[0204]Powder of a coprecipitated precursor was synthesized in the same manner as in Example I-1 except for the following procedures. That is, the nickel sulfate aqueous solution, a cobalt sulfate aqueous solution and the manganese sulfate aqueous solution were mixed with each other so that a molar ratio of nickel, cobalt and manganese was adjusted to Ni:Co:Mn=0.35:0.05:0.60 to give a mixture solution.
[0205]It was found that the coprecipitated precursor was a carbonate precursor compound represented by the formula: (Ni0.35Co0.05Mn0.60)CO3. Lithium carbonate powder was weighed so that a molar ratio of lithium to the coprecipitated precursor was adjusted to Li / (Ni+Co+Mn)=1.25, and the lithium carbonate powder was fully mixed with the coprecipitated precursor to give a mixture. The mixture was subjected to calcination under the oxidizing atmosphere at 850° C. for 5 hours in the electric furnace to give a positive electrode active material.
example i-3
[0207]Powder of a coprecipitated precursor was synthesized in the same manner as in Example I-1 except for the following procedures. That is, the nickel sulfate aqueous solution, the cobalt sulfate aqueous solution and the manganese sulfate aqueous solution were mixed with each other so that a molar ratio of nickel, cobalt and manganese was adjusted to Ni:Co:Mn=0.310:0.055:0.635 to give a mixture solution.
[0208]It was found that the coprecipitated precursor was a carbonate precursor compound represented by the formula: (Ni0.310Co0.055Mn0.635)CO3. Lithium carbonate powder was weighed so that a molar ratio of lithium to the coprecipitated precursor was adjusted to Li / (Ni+Co+Mn)=1.375, and the lithium carbonate powder was fully mixed with the coprecipitated precursor to give a mixture. The mixture was subjected to calcination under the oxidizing atmosphere at 880° C. for 5 hours in the electric furnace to give a positive electrode active material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com