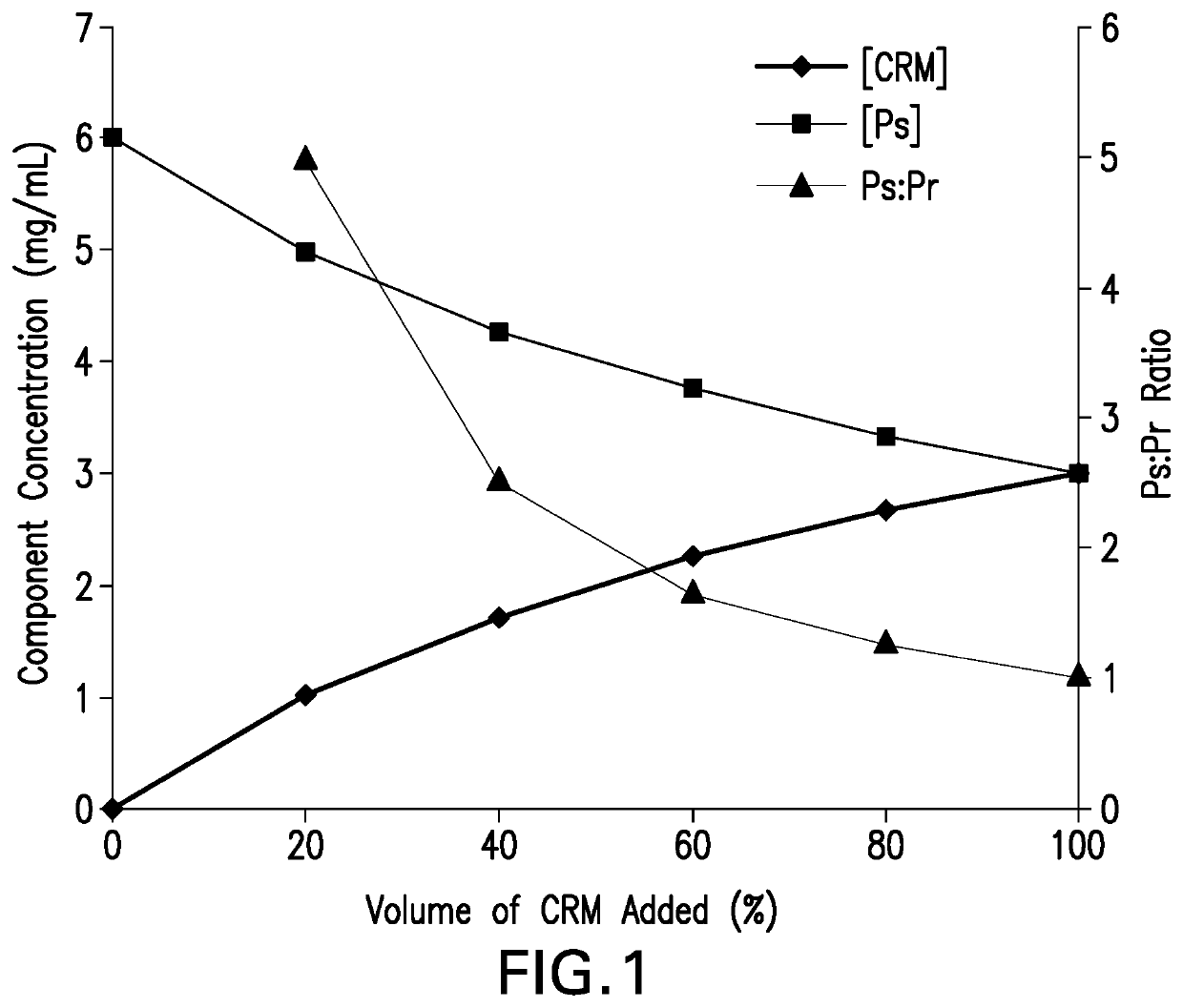
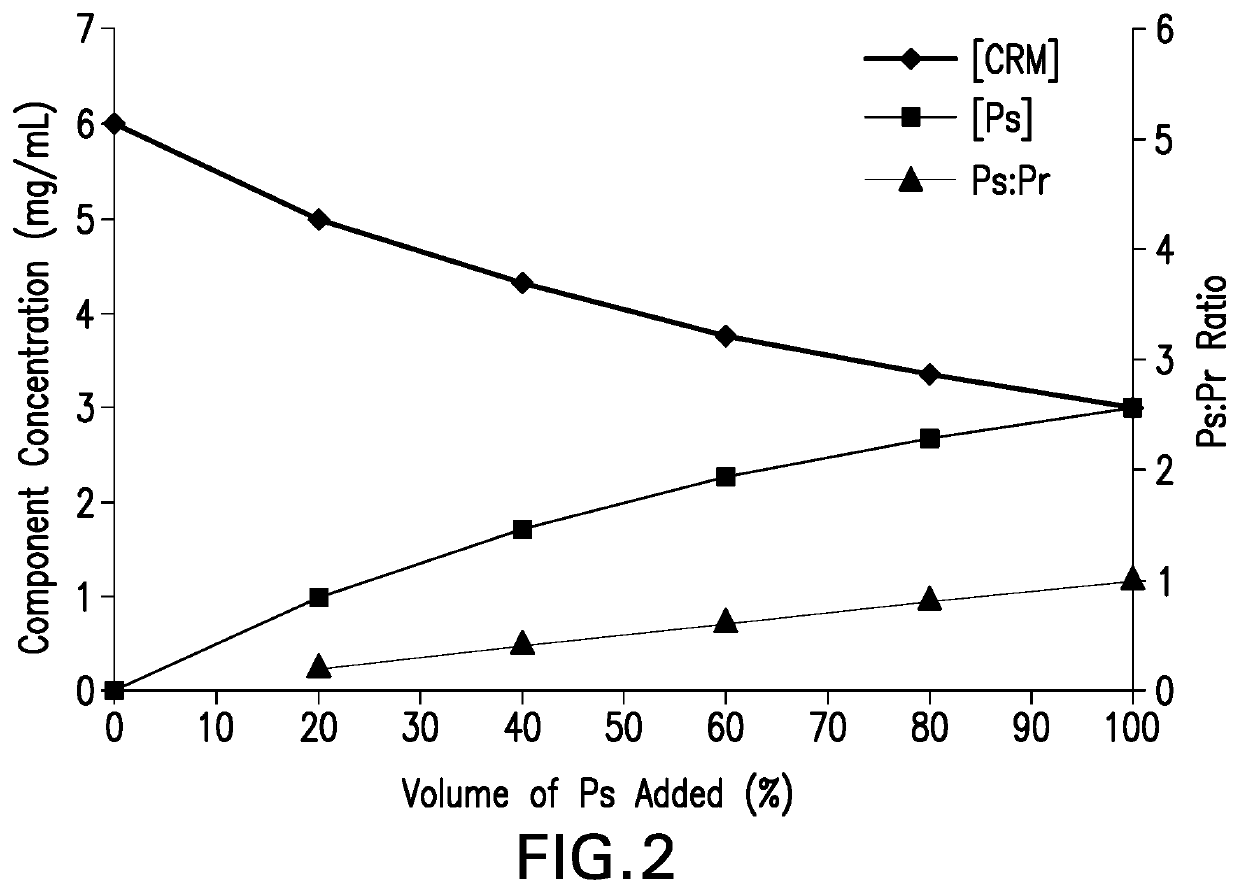
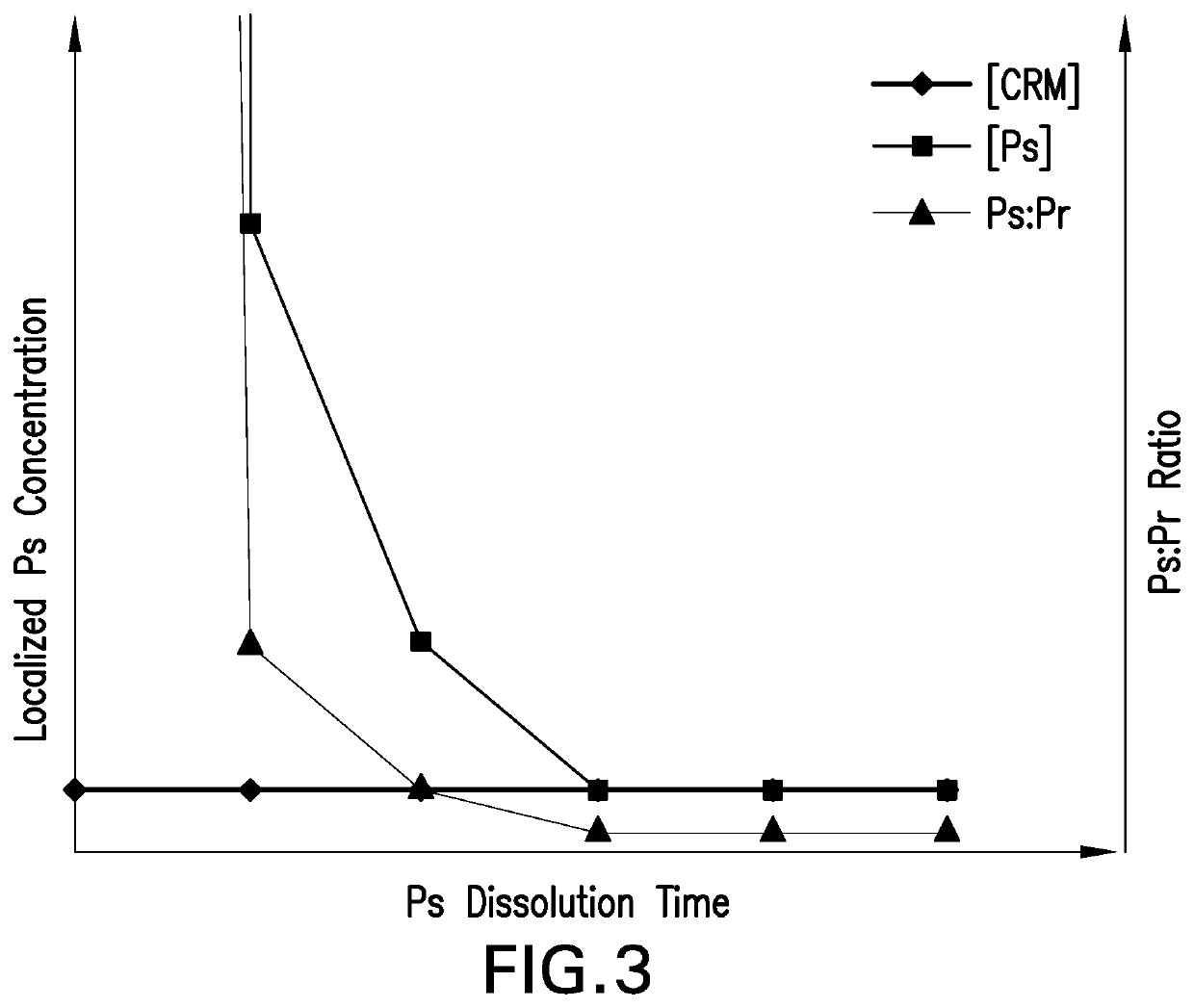
Methods for producing streptococcus pneumoniae capsular polysaccharide carrier protein conjugates
a technology of capsular polysaccharide and pneumococcal polysaccharide, which is applied in the direction of pharmaceutical delivery mechanism, inorganic non-active ingredients, antibody medical ingredients, etc., can solve the problems of lyophilization preclude the ability to optimize individual formulations and cycle parameters, and the complication of pneumococcal diseases can be significant, etc., to achieve the ability to optimize individual formulations and cycle parameters, and facilitate handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of S. Pneumoniae Capsular Polysaccharides 6A, 6B, 7F, 18C, 19A, 19F, and 23F
[0188]Methods of culturing pneumococci are well known in the art. See, e.g., Chase, 1967, Methods of Immunology and Immunochemistry 1:52. Methods of preparing pneumococcal capsular polysaccharides are also well known in the art. See, e.g., European Patent No. EP0497524. Isolates of pneumococcal subtypes are available from the American Type Culture Collection (Manassas, Va.). The bacteria are identified as encapsulated, non-motile, Gram-positive, lancet-shaped diplococci that are alpha-hemolytic on blood-agar. Subtypes can be differentiated on the basis of Quelling reaction using specific antisera. See, e.g., U.S. Pat. No. 5,847,112.
[0189]Cell banks representing each of the S. pneumococcus serotypes present are obtained from the Merck Culture Collection (Rahway, N.J.) in a frozen vial. A thawed seed culture is transferred to the seed fermentor containing a pre-sterilized growth media appropriate f...
example 2
[0191]Polysaccharide size reduction and activation is performed as follows.
[0192]About 6 g of purified pneumococcal capsular polysaccharide (Ps) powder is dissolved in water for injection (WFI) to a target concentration of approximately 4 g / L at room temperature. The solution is then 0.45-micron filtered to reduce bioburden. The Ps concentration of the filtered Ps solution is determined by HPSEC UV-MALS-RI.
[0193]For all serotypes except serotypes 18C and 19A, the solution is diluted to approximately 2.5 g / L and then homogenized to reduce the molecular mass of the Ps using a GEA-Niro Soavi Panda 2K homogenizer. All serotypes, except serotype 19A, were homogenized to reduce the molecular mass of the polysaccharide. Serotype 19A was not size reduced due to its relatively low starting size. Homogenization pressure and number of passes through the homogenizer were controlled to serotype-specific targets (150-1000 bar; 4-7 passes) to achieve a serotype-specific molecular mass. Size-reduce...
example 3
[0200]Preparation of CRM197 Carrier Protein may be performed as follows.
[0201]Frozen, purified CRM197, obtained through expression in Pseudomonas fluor escens as previously described (See International Patent Application Publication No. WO 2012 / 173876), is diafiltered against 10 diavolumes of 2 mM phosphate, pH 7.2 buffer using a 5 kDa NMWCO tangential flow ultrafiltration membrane and 0.22-micron filtered. In particular embodiments, 5 mM potassium phosphate, pH 6.4 (5 mM sodium phosphate, pH 7.0 is used for serotype 18C. The diafiltered solution is diluted in water with sucrose for a final concentration of between 1.0 and 5.3% (w / v) and lyophilized to produce dried CRM197 having a final moisture content of 6% or less. Table 2 below provides representative sucrose concentrations for CRM197 for conjugation against particular serotype polysaccharides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com