Ligands to gm-csf or gm-csf-receptor for use in treatment of a haematologic malignancy in a patient having undergone allo-hct
a technology of gm-csf and receptor, which is applied in the direction of immunodeficiency, drug composition, peptide, etc., can solve the problems of increased relapse rate, decreased graft-versus-leukemia activity, and inability to identify specific soluble mediators, so as to prevent, inhibit or reduce the severity of graft-versus-host disease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Tissue-Infiltrating Donor T Cells Produce GM-CSF and IFNγ Following MHC-Mismatched HCT
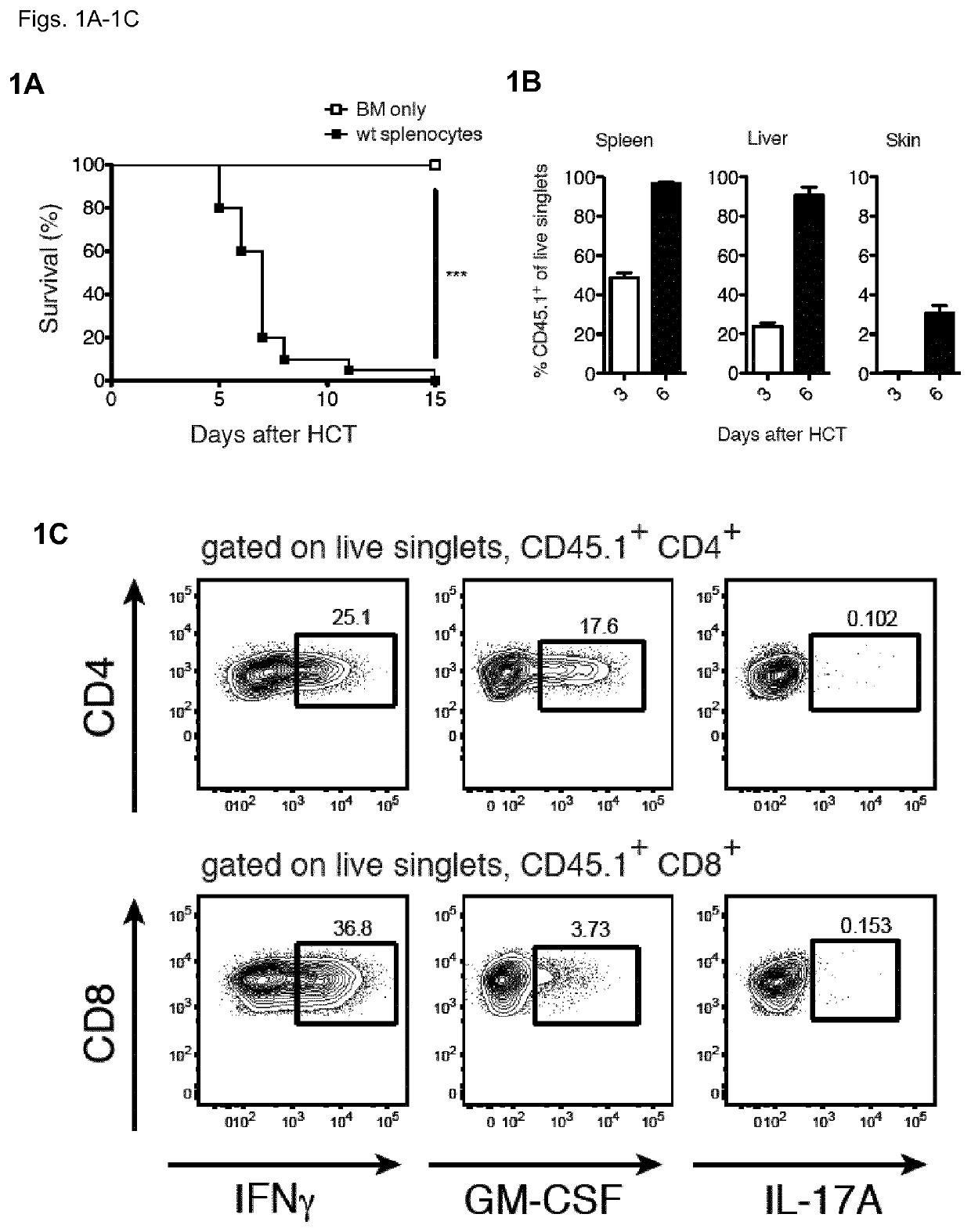
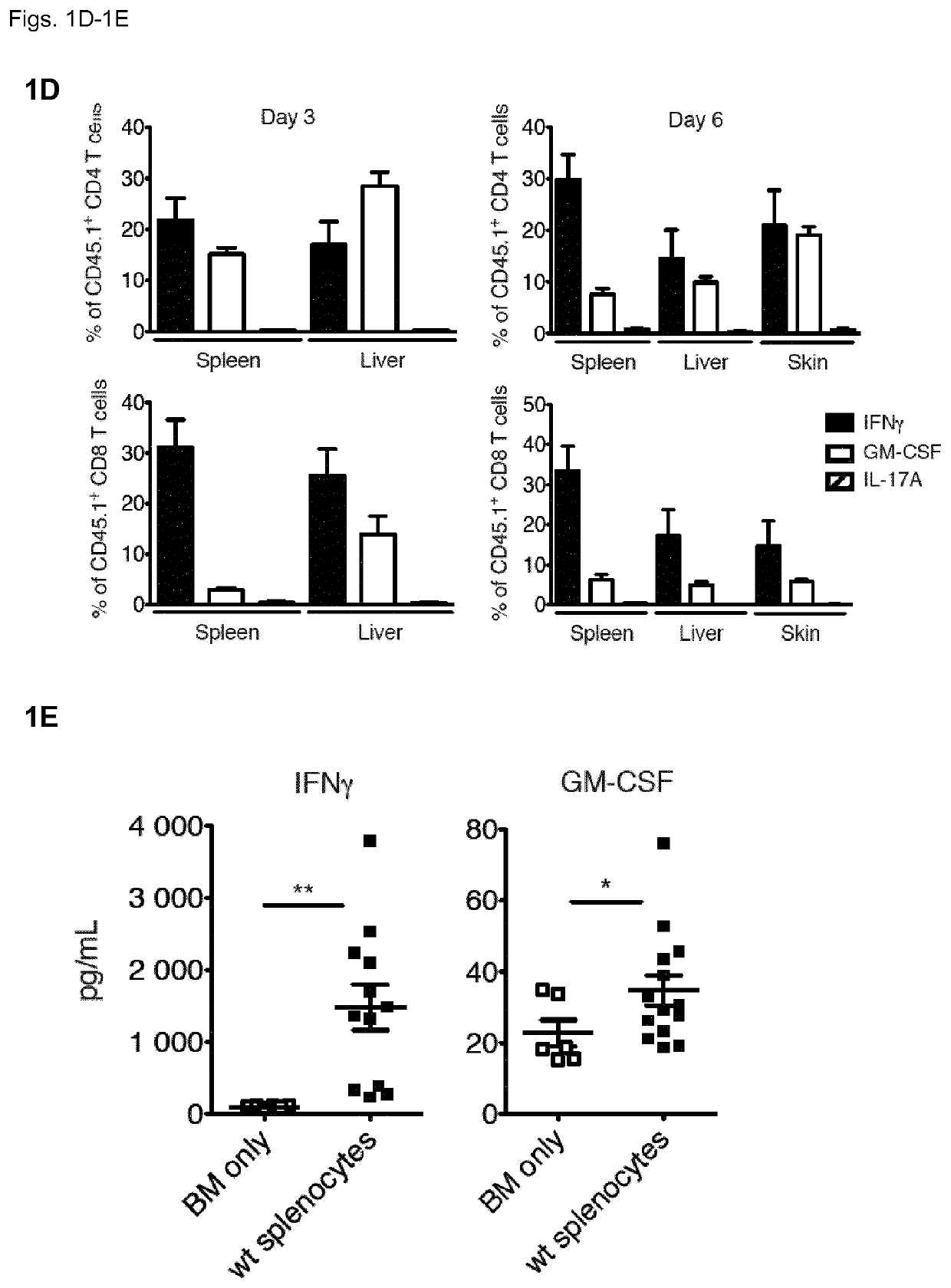
[0131]To understand the contribution of T cell-derived GM-CSF to acute GvHD, the inventors first employed a model of MHC-mismatched HCT. The inventors lethally-irradiated wild-type (WT) BALB / c CD45.2+ mice (MHC haplotype H2d) and then intravenously injected them with T cell-depleted (TCD) bone marrow (BM) cells from WT CD45.1 C57BL / 6 (B6) mice (MHC haplotype H2b), with or without B6 CD45.1+ splenocytes, which served as a source of mature T cells. Recipients of BM plus splenocytes developed fatal GvHD between days 3 and 6 after allo-HCT (FIG. 1A), which coincided with the reconstitution and expansion of donor CD45.1+ cells in the spleen, liver and skin (FIG. 1B). Within the CD45.1+ donor compartment, T cell populations contained high frequencies of GM-CSF- and IFNγ-producing cells, seen first in the liver (FIG. 1C) and spleen (FIG. 1D) from day 3 after allo-HCT, then in the skin from day 6 (FIG. 1D)...
example 2
GM-CSF Production by Allogeneic T Cells is Essential for GvHD Pathology
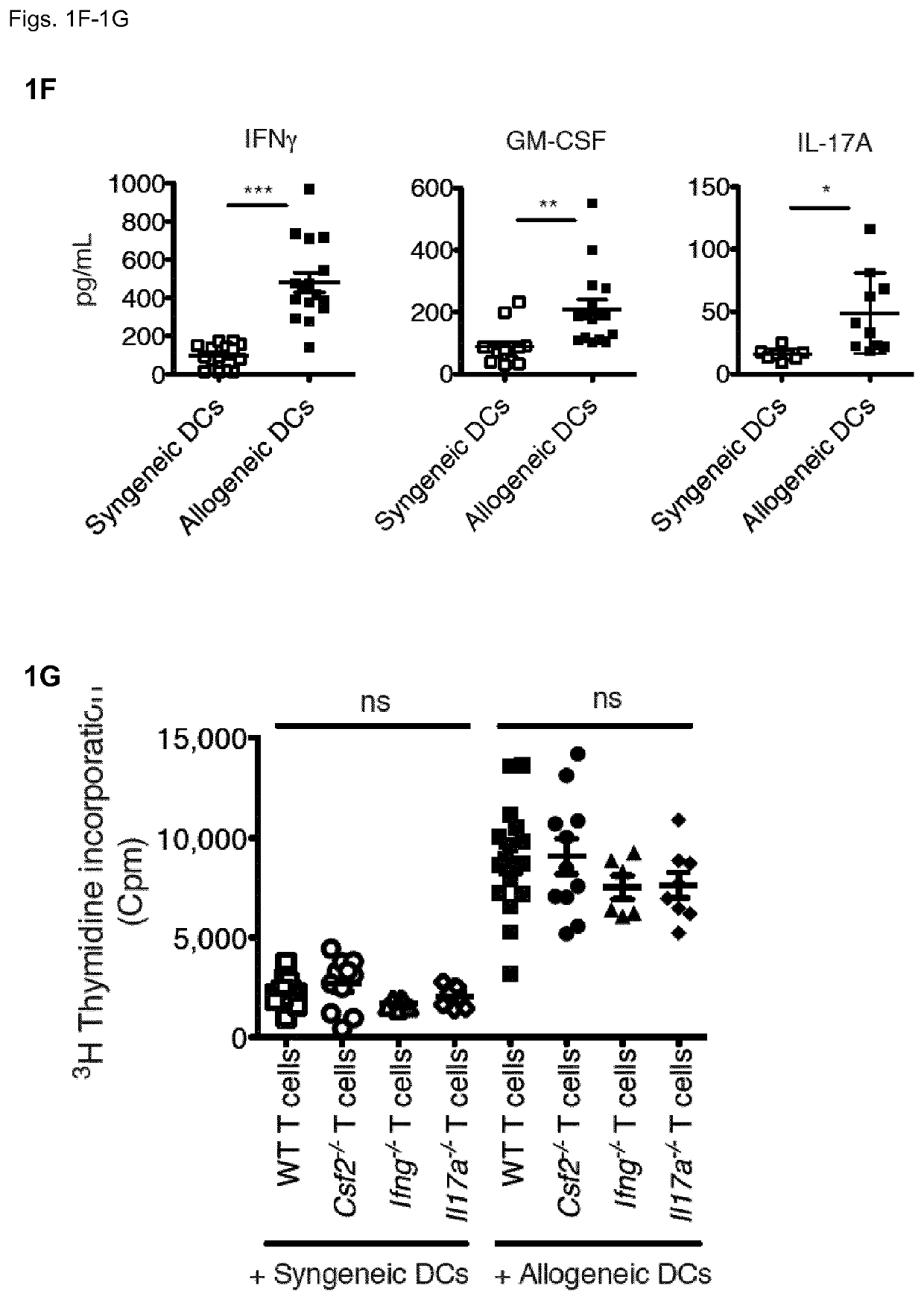
[0132]Given the production of GM-CSF and IFNγ by allo-reactive TH cells, the inventors assessed the relevance of these cytokines in determining severity of acute GvHD. As above, the inventors lethally-irradiated WT BALB / c mice and then compared the outcomes of injecting TCD-BM cells from WT B6 mice, with or without splenocytes from WT B6 mice, or from B6 mice lacking GM-CSF (Csf2− / −), IFNγ (Ifng− / −) or IL-17 (II17a− / −). Mice receiving BM plus Csf2− / − splenocytes were protected from lethal GvHD up to 20 days post allo-HCT, while all mice treated with BM plus WT splenocytes had died by day 15 after HCT (FIG. 2A). Injecting mice with BM plus Ifng− / − or II17a− / − splenocytes also significantly affected the fatal GvHD kinetic, but still almost 100% of these mice died by day 20 (FIG. 2A).
[0133]As donor splenocyte preparations contain multiple different immune cell types, the inventors then asked whether T cells were ind...
example 3
GM-CSF Mediates GvHD Lethality Through Donor-Derived Myeloid Cells
[0139]Following lethal conditioning, radio-sensitive host antigen-presenting cells (APCs) are lost within the first days after HCT and substituted by APCs from the donor. Whereas host APCs are required for the priming phase of GvHD, donor APCs play less of a role in the induction of the disease but may be involved in perpetuating tissue injury. To delineate whether the pathologically relevant GM-CSF-responsive cell type(s) are of donor or host-origin, the inventors used Csf2rb− / − mice lacking the beta subunit of the GM-CSFR as donors or hosts of HCT. GM-CSFR deficiency in the recipient compartment did not influence GvHD survival (FIG. 9A). In contrast, the transfer of BM from Csf2rb− / − mice resulted in dramatically delayed mortality (FIG. 9B), phenocopying the transfer of Csf2− / − splenocytes (FIG. 2A). The inventors used unsupervised non-linear dimensionality reduction (t-SNE) to identify and visualize the GM-CSF-resp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com