Microbubble and nanobubble expansion using perfluorocarbon nanodroplets for enhanced ultrasound imaging and therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
lass="d_n">[0055]An overview of exemplary embodiments of the present disclosure will be presented initially, followed by further discussion of specific aspects.
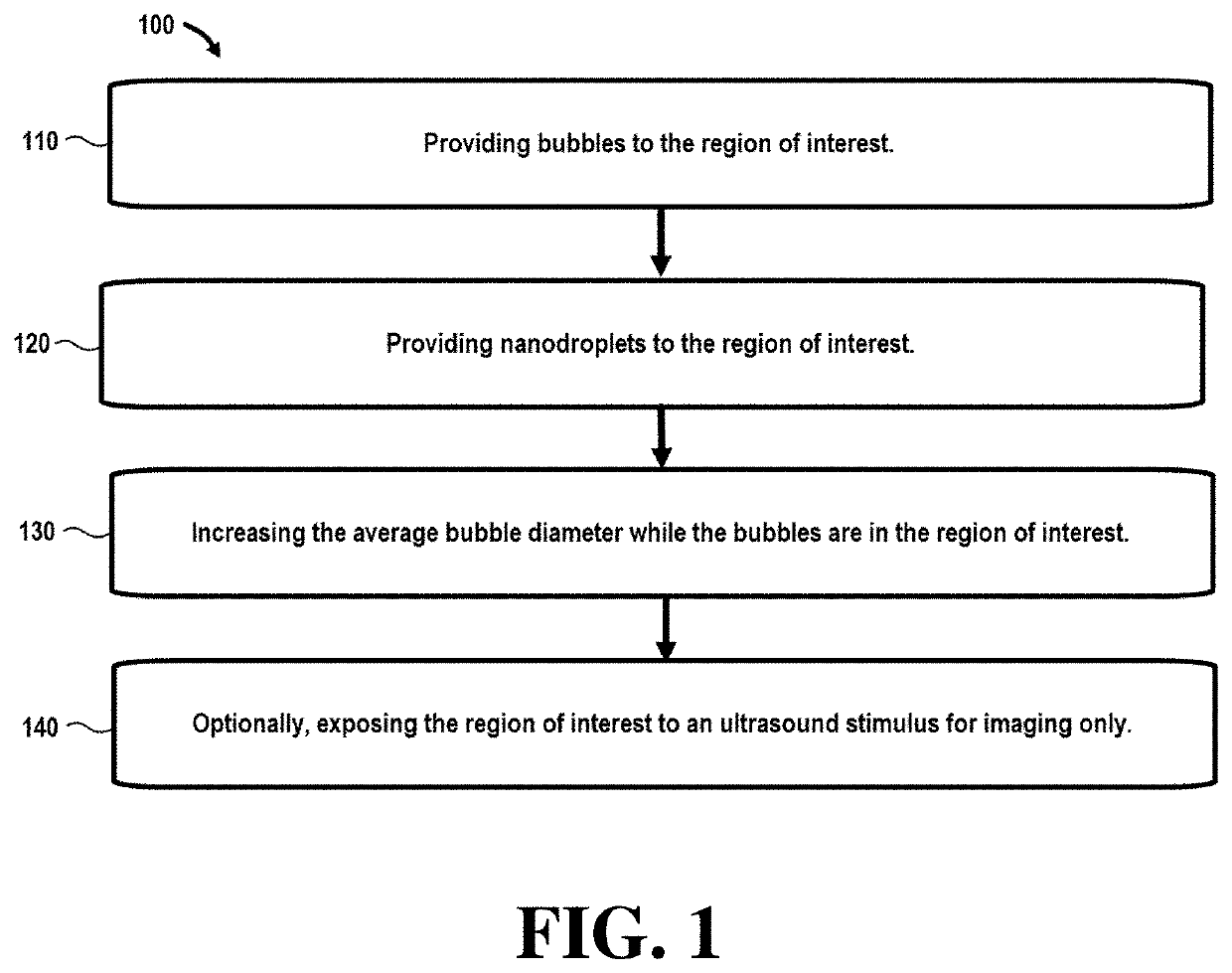
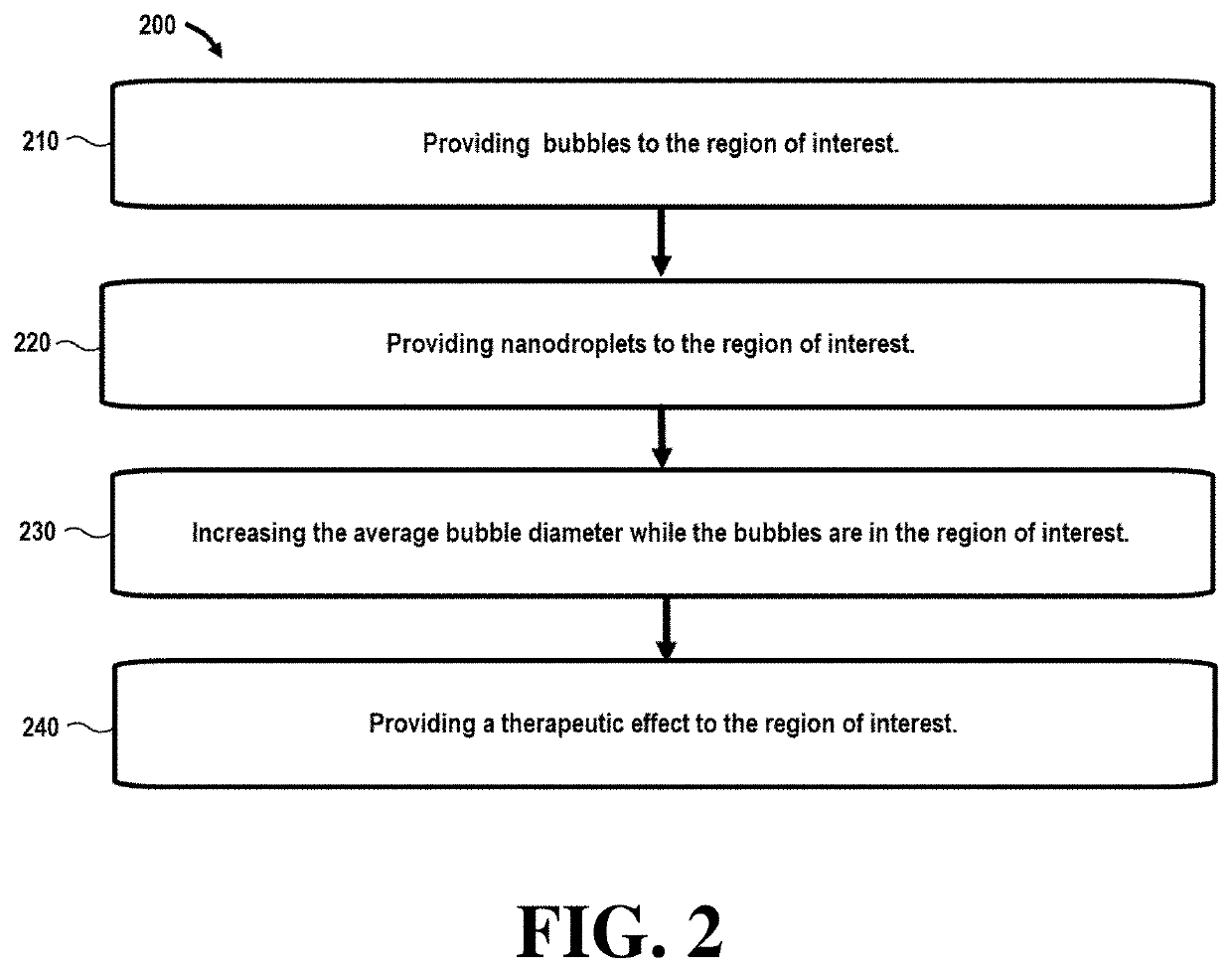
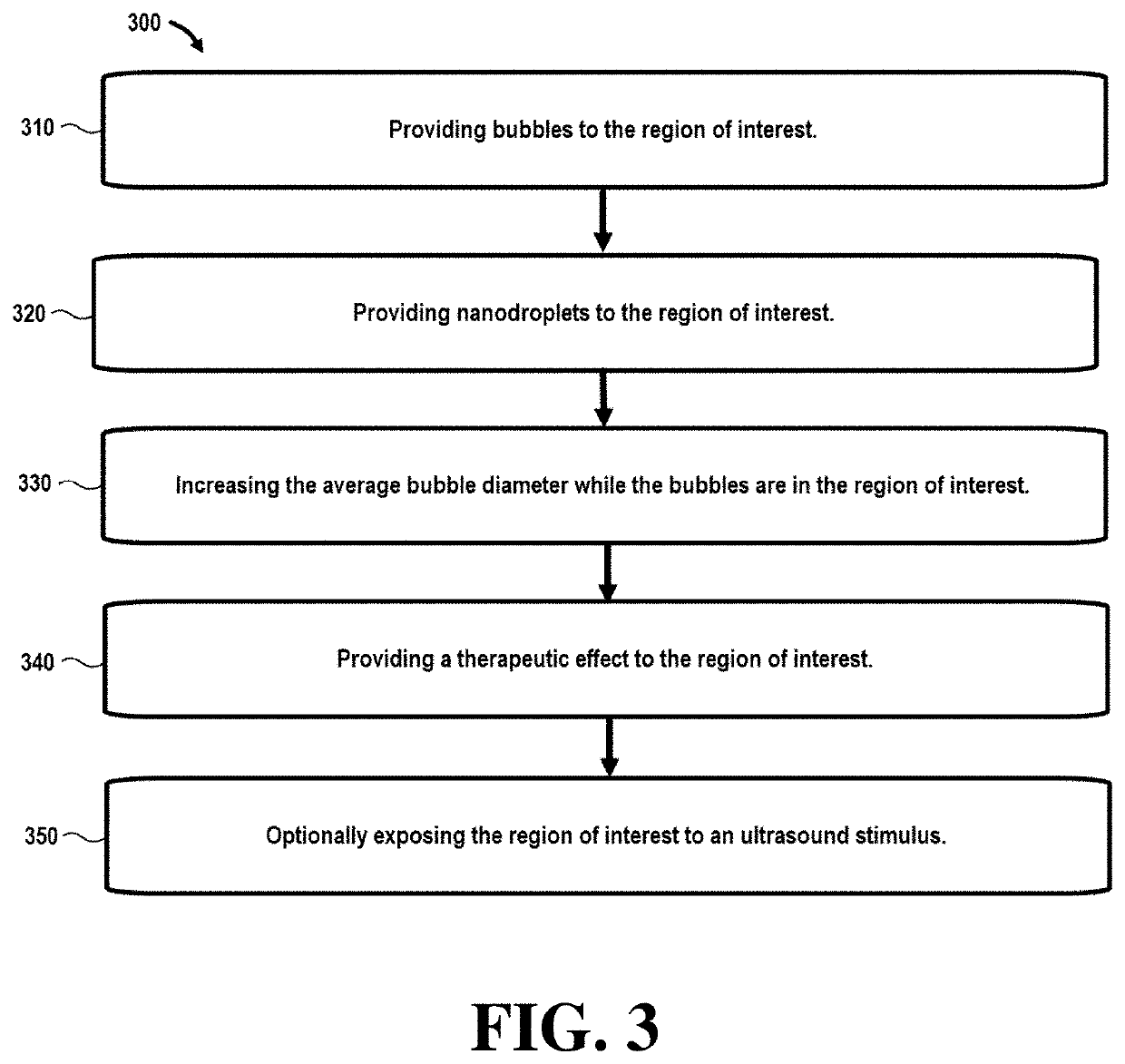
[0056]Referring initially to FIG. 1, a flowchart 100 illustrates steps in a method that can be used in ultrasound imaging techniques. In this embodiment, step 110 comprises providing bubbles to the region of interest. In certain embodiments, the bubbles may be nanobubbles or microbubbles with an average bubble diameter between around 200-600 nm (nanobubbles) and 1-10 μm (microbubbles).
[0057]To circumvent the challenges of ADV for effective gas embolotherapy, the inventors took inspiration from a biological process and the second law of thermodynamics: the process by which PFC is eliminated from the body by exhalation (25). When PFC liquid emulsions or PFC gas-filled MBs traverse the alveolar capillaries, PFC transfers to the air-filled alveolar space because of the large partial pressure gradient (1). In addition, since liqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com