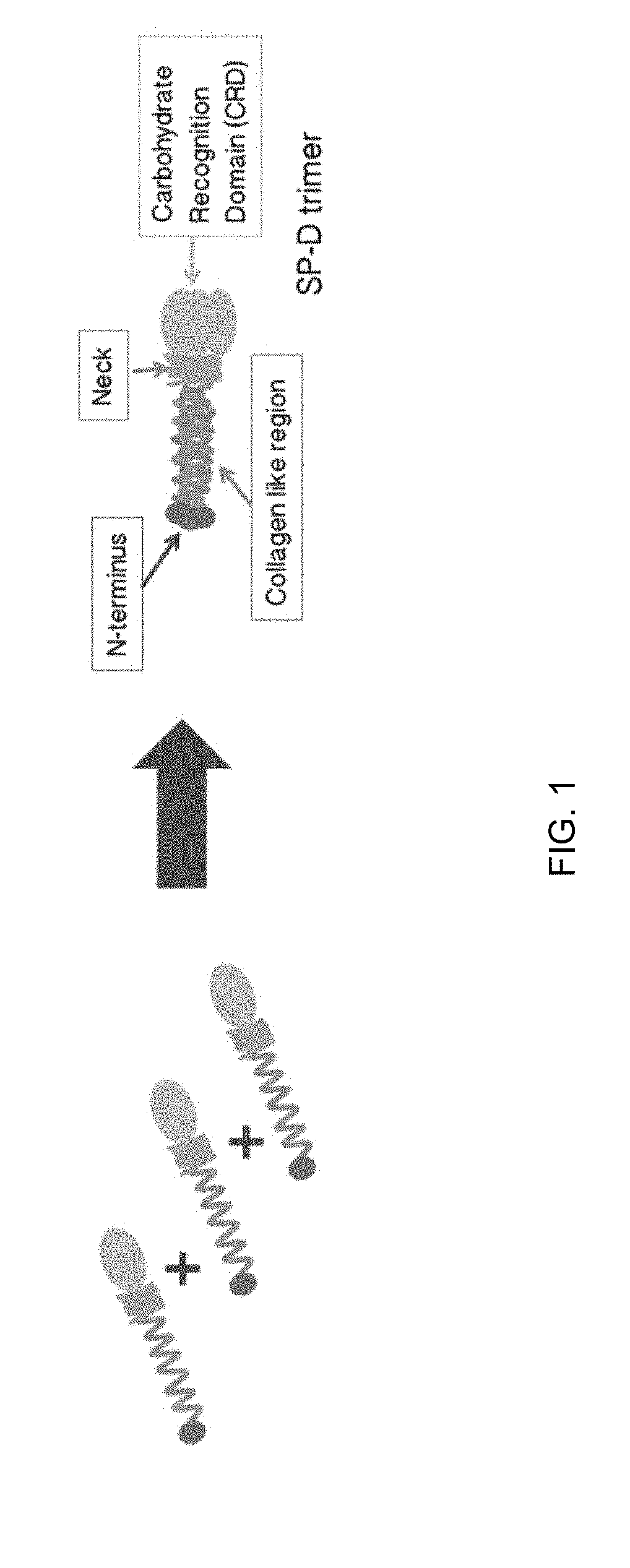
Methods and compositions for preparing surfactant protein d (sp-d)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
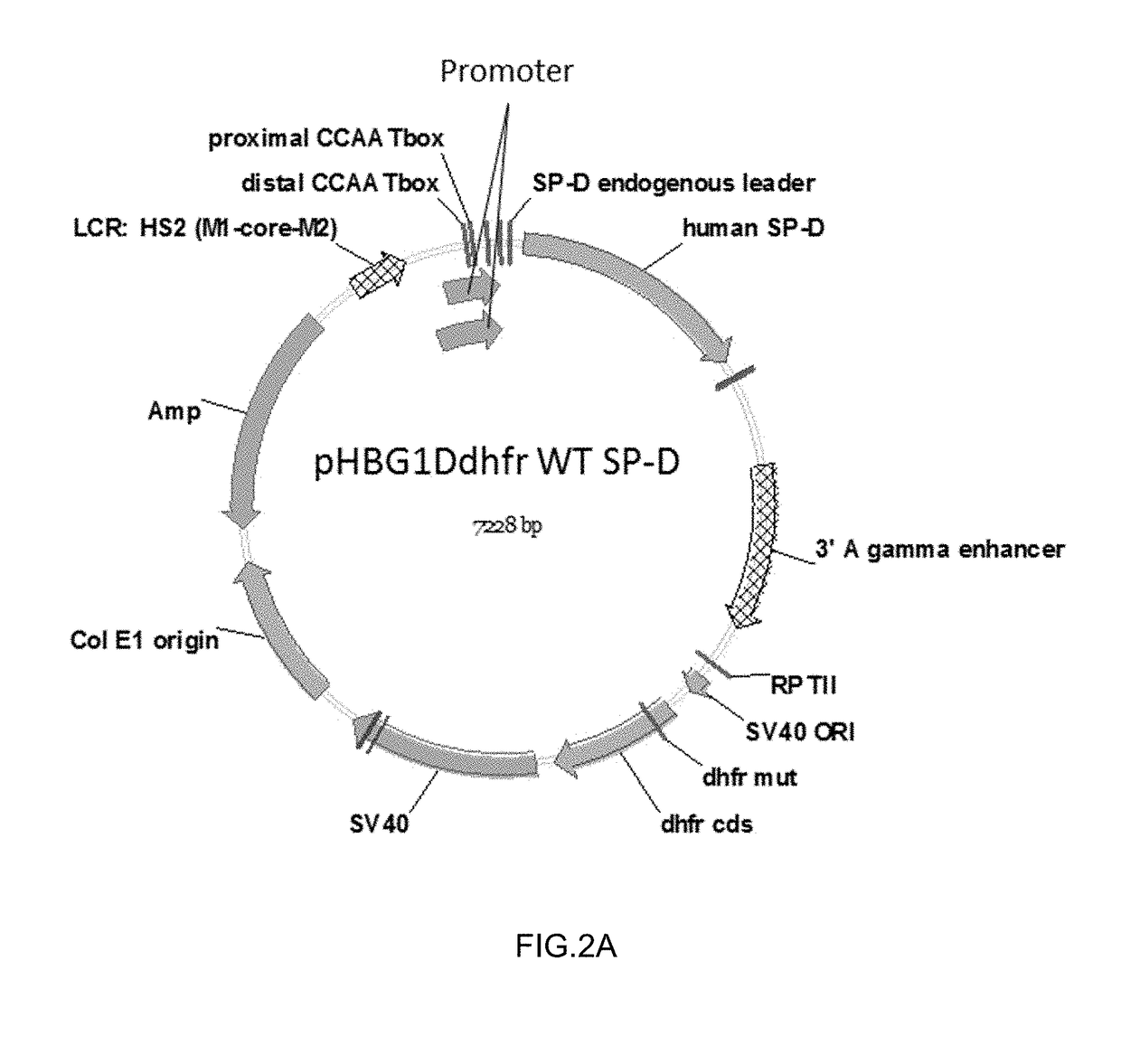
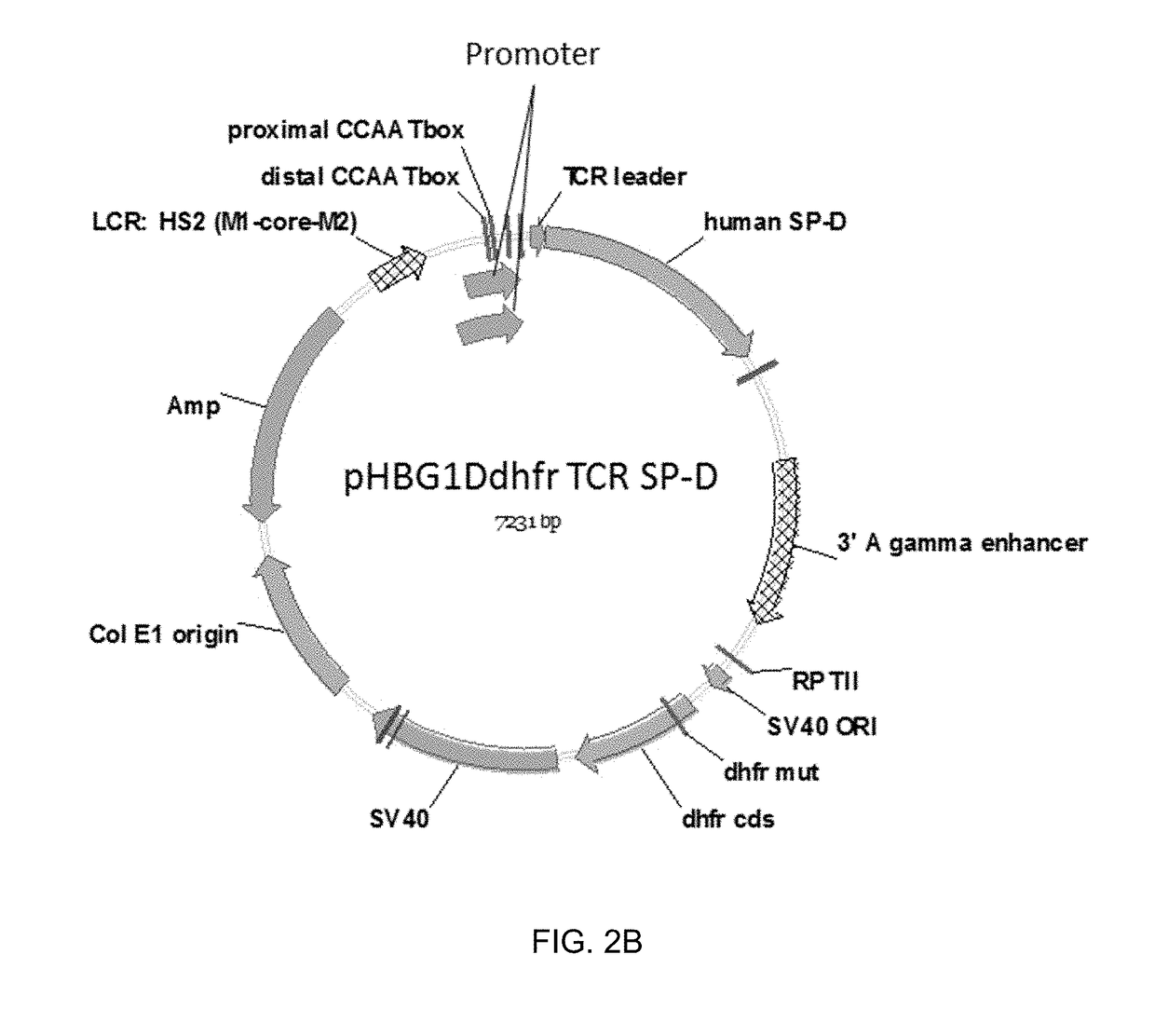
Construction of SP-D Expression Vectors
[0104]Two expression vectors were developed for expression of human SP-D in human mammalian cells. One vector included a wild-type human SP-D leader / signal sequence; the other vector included a human T-cell receptor (TCR) leader / signal sequence. The TCR leader sequence was selected for one of the expression vectors because the proteins would be expressed in human myeloid leukemia cells which would be expected to secrete proteins with a TCR leader sequence at a high efficiency.
[0105]Polynucleotides encoding a human SP-D polypeptide and including either the wild-type human SP-D leader / signal sequence or the human T-cell receptor (TCR) leader / signal sequence were synthesized by GENEART (ThermoFisher Scientific). Each polynucleotide included Xba I (5′ end) and Hind III (3′ end) restriction sites for cloning purposes, and a Kozak consensus sequence. Each polynucleotide was excised from a GENEART (ThermoFisher Scientific) delivery vector by Hind III / ...
example 2
Expression of SP-D in Mammalian Cell Lines
[0106]Prior to transfection, expression vectors were linearized with Pvu I and purified with phenol / chloroform, and trichlormethan / chloroform. Cell lines were transfected with 7-8 μg of a linearized expression vector using NUCLEOFECTION according to the manufacturer's instructions (AMAXA NUCLEOFECTOR TECHNOLOGY; Lonza, Cologne, Germany). The following cell lines were transfected with expression vectors: NM-H9D8 (DSM ACC 2806); NM-H9D8-E6Q12 (DSM ACC 2856); and NM-F9 (DSM ACC2606).
[0107]Pools of cells expressing SP-D were selected using 25 nM methotrexate (MTX) and increasing the concentration to 50 nM MTX. To obtain cells with increasing levels of SP-D expression, the concentration of MTX was increased in steps from 100 nM to 200 nM to 400 nM MTX. The productivity of SP-D producing cells was determined by SP-D specific ELISA (BioVendor GmbH, Germany, Cat# RD194059101) according to the manufacturer's instructions, and / or Dot-Blot analysis usi...
example 3
Culturing SP-D Expressing Cell Lines
[0110]Cells were cultured in a serum-free chemically defined gene therapy medium (GTM) (Glycotope GmbH, Germany). See e.g., U.S. Pat. No. 9,359,427 which is incorporated by reference in its entirety for a description of the GTM culture media. Perfusion process cultures were initiated with 1×GTM, and then modified to 2×GTM. Cells were maintained in exponential growth phase by splitting every 2 to 3 days to a cell concentration of 1×105 to 3×105 cells / mL in T flasks (25 cm2, 3 to 6 mL suspension volume, TPP, Germany) and were incubated at 37° C., 98% humidity and 8% CO2 (Integra Biosciences IBS, Biosafe plus, Switzerland or Thermo / Heraeus BBD 6220, Germany). Cell expansion was carried out using T flasks (75 cm2, 12 to 30 mL Volume; 150 cm2, 50 to 150 mL) and Spinner flasks (100 mL to 1000 mL, Integra Biosciences IBS, Cellspin, Switzerland).
[0111]General cultivation parameters: media were inoculated with 2.0×105 cells / mL. Continuous operation was ena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com