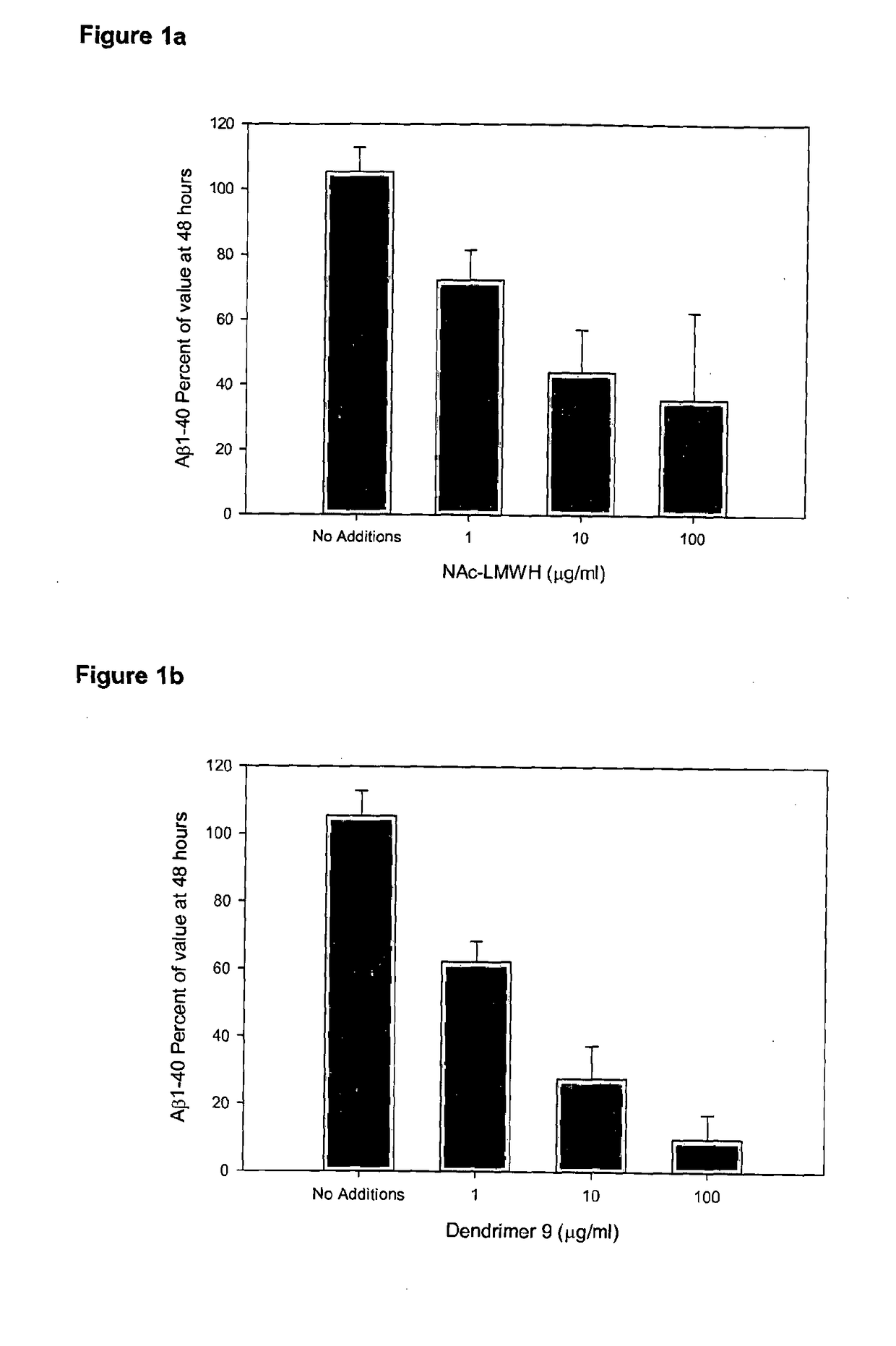
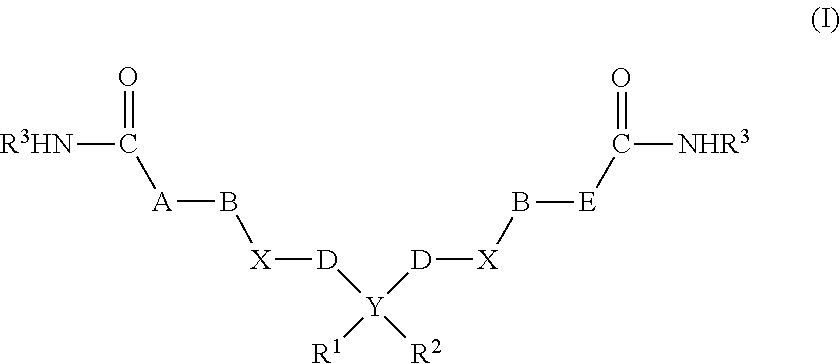
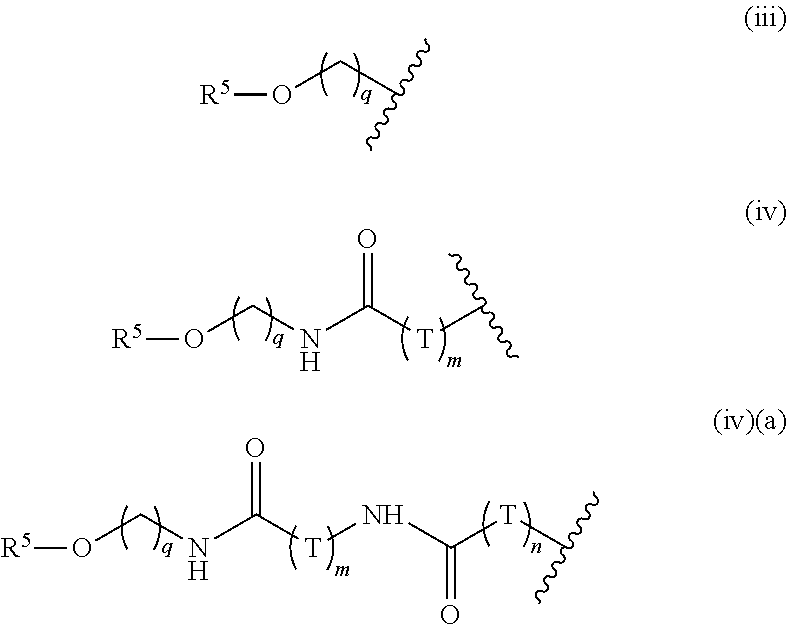
Saccharide Dendritic Cluster Compounds as Inhibitors of Bace-1
a dendritic cluster and bace-1 technology, applied in the field of dendritic compounds, can solve the problems of difficult synthesis of long linear heparan sulfate mimics, and difficulty in synthesis, and achieve the effect of reducing the amount of a1-40
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds
Example 1.1
Synthesis of tetra-succinimidyl ester
[0165]
[0166]Preparation of 1′
[0167]Tetranitrile precursor (Ref.1, Hukkämaki, J.; Pakkanen, T. T. Journal of Molecular Catalysis A: Chemical 2001, 174, 205-211) is prepared via Michael-type addition of acrylonitrile to pentaerythritol. Acidic hydrolysis of tetranitrile (Ref. 2, Newcombe, G. R.; Mishra, A; Moorfield, C. N. J. Org. Chem. 2002, 67, 3957-3960) furnishes the tetraacid. Tetraacid (1.0 g, 2.35 mmol) is dissolved in dry DMF (15 mL). N-Hydroxysuccinimide (1.62 g, 14.14 mmol) and 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC, 2.71 g, 14.14 mmol) are added to the reaction mixture at room temperature and stirring continued for 24 hrs. The mixture is diluted with DCM and washed with water, then with diluted HCl and water, dried over magnesium sulfate and concentrated. The residue is purified by flash chromatography on silica gel eluting with EtOAc followed by EtOAc:MeOH, 19:1→9:1→7:1→4:1 to g...
example 1.2
Synthesis of di-succinimidyl ester
[0183]
[0184]Preparation of 14′
[0185]3,6,9-Trioxaundecanedioic acid (500 mg, 2.25 mmol) is dissolved in dry DMF (10 mL). N-Hydroxysuccinimide (785 mg, 6.75 mmol, 3 eq.), 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC, 1.32 g, 6.75 mmol, 3 eq.) and N,N-diisopropylethylamine (2.38 mL, 13.5 mmol) followed by benzyl 8-aminooctanate (1.68 g, 6.75 mmol, 3 eq.) are added to the reaction mixture at room temperature and stirring continued for 4 hrs. The mixture is diluted with DCM and washed with water, then with diluted HCl and water, dried over magnesium sulfate and concentrated. The residue is dissolved in hot EtOAc, the crystals are filtered off and discharged. The mother liquor is concentrated and the residue is purified by flash chromatography on silica gel eluting with Chloroform:EtOAc:MeOH, 5:2:0.3 to afford the di-benzyl ester (10, 1.1 g, 1.61 mmol, 71%). 13C-NMR (125 MHz, CDCl3) δ 173.4, 169.9, 136.1, 128.4, 128.3, 128.1, 127.8, 12...
example 1.3
Synthesis of Dendritic Cluster Compounds
[0190]Preparation of 6e (Scheme 8)
[0191]Thioglycoside donor (WO 2012 / 121617) (1 g, 2.2 mmol) and Cbz-protected hexa-amino alcohol (Chipowsky, S.; Lee, Y. C. Carb. Res., 1973, 31, 339-346) (1 g, 4.3 mmol, 2.0 eq) are dissolved in anhydrous dichloromethane (20 mL) and cooled to −15° C. and powdered molecular sieves (4 Å) are added. After 10 min N-iodosuccinimide (836 mg, 3.7 mmol, 1.7 eq) and silver trifluoromethanesulfonate (281 mg, 1 mmol, 0.5 eq) are added. The reaction mixture is allowed to warm up to room temperature over 1 h. The mixture is diluted with ethyl acetate and filtered through celite. The filtrate is washed with a 1:1 mixture of saturated aq. sodium bicarbonate and aq. thiosulfate (30%), washed with saturated aq. sodium chloride, dried over magnesium sulfate and concentrated. The residue is purified by silica gel chromatography (EtOAc:petroleum ether, 1:2) to furnish the glycoside 6e as a syrup (1.35 g, 2.0 mmol, 93%), TLC (EtOA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com