Methods of treating metabolic disorders associated with lipodystrophies and defects in insulin production or signaling
a metabolic disorder and lipodystrophic disease technology, applied in the field of metabolic disorders associated with insulin resistance, can solve the problems of many cell types not responding, and achieve the effect of enhancing the biological properties of the varian
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vivo Administration of FGF21 in Type 1 Diabetes Mouse Models
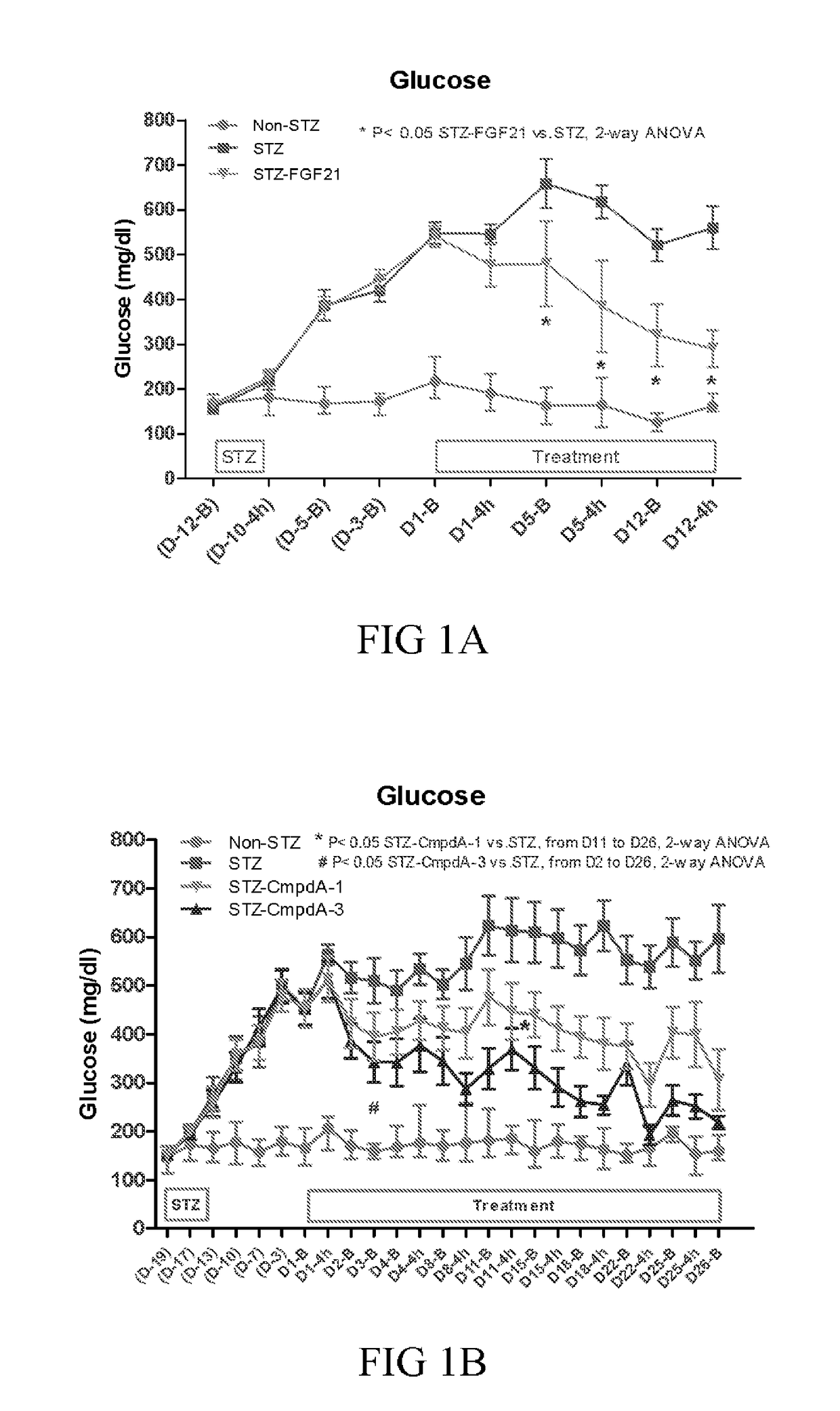
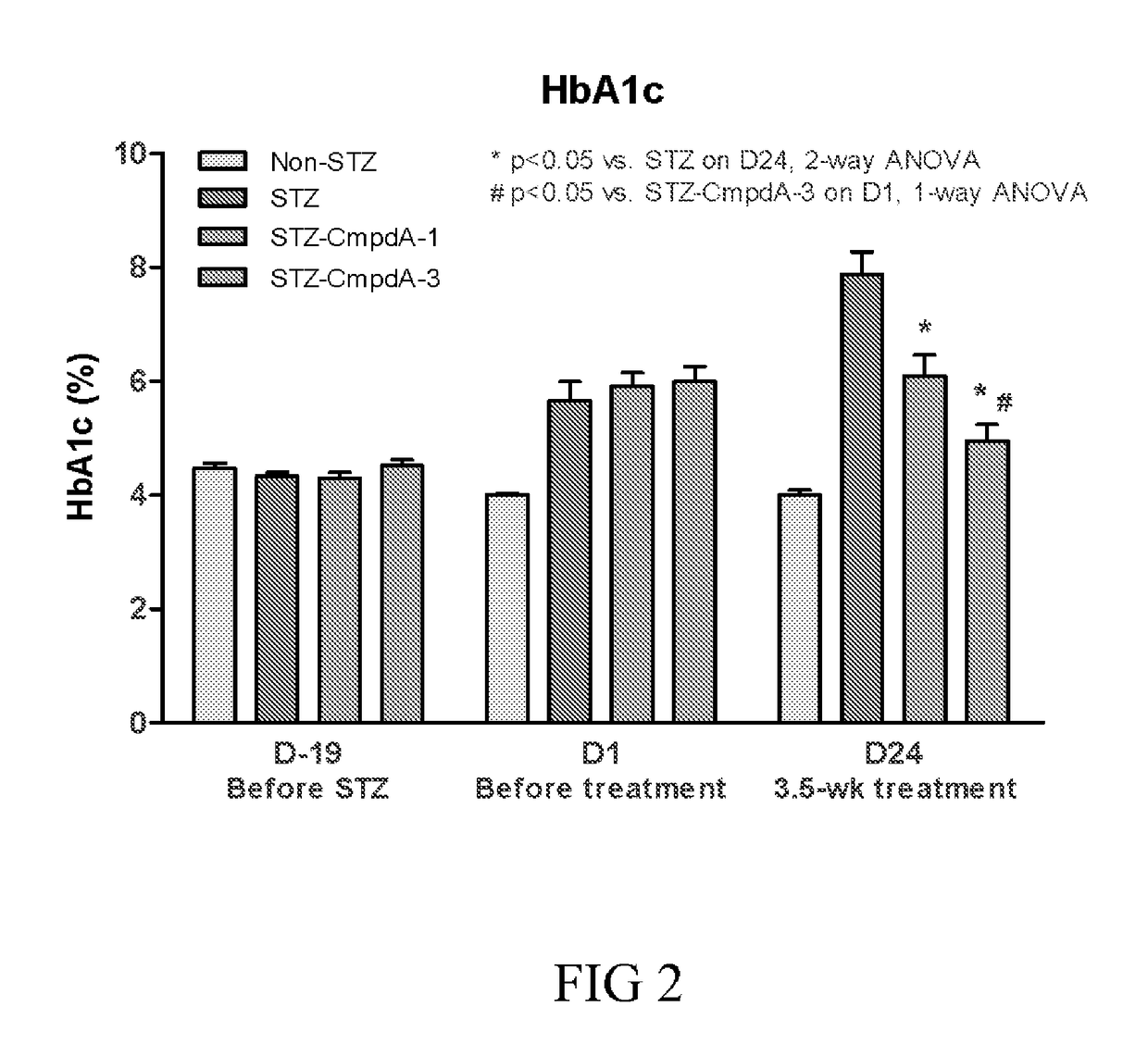
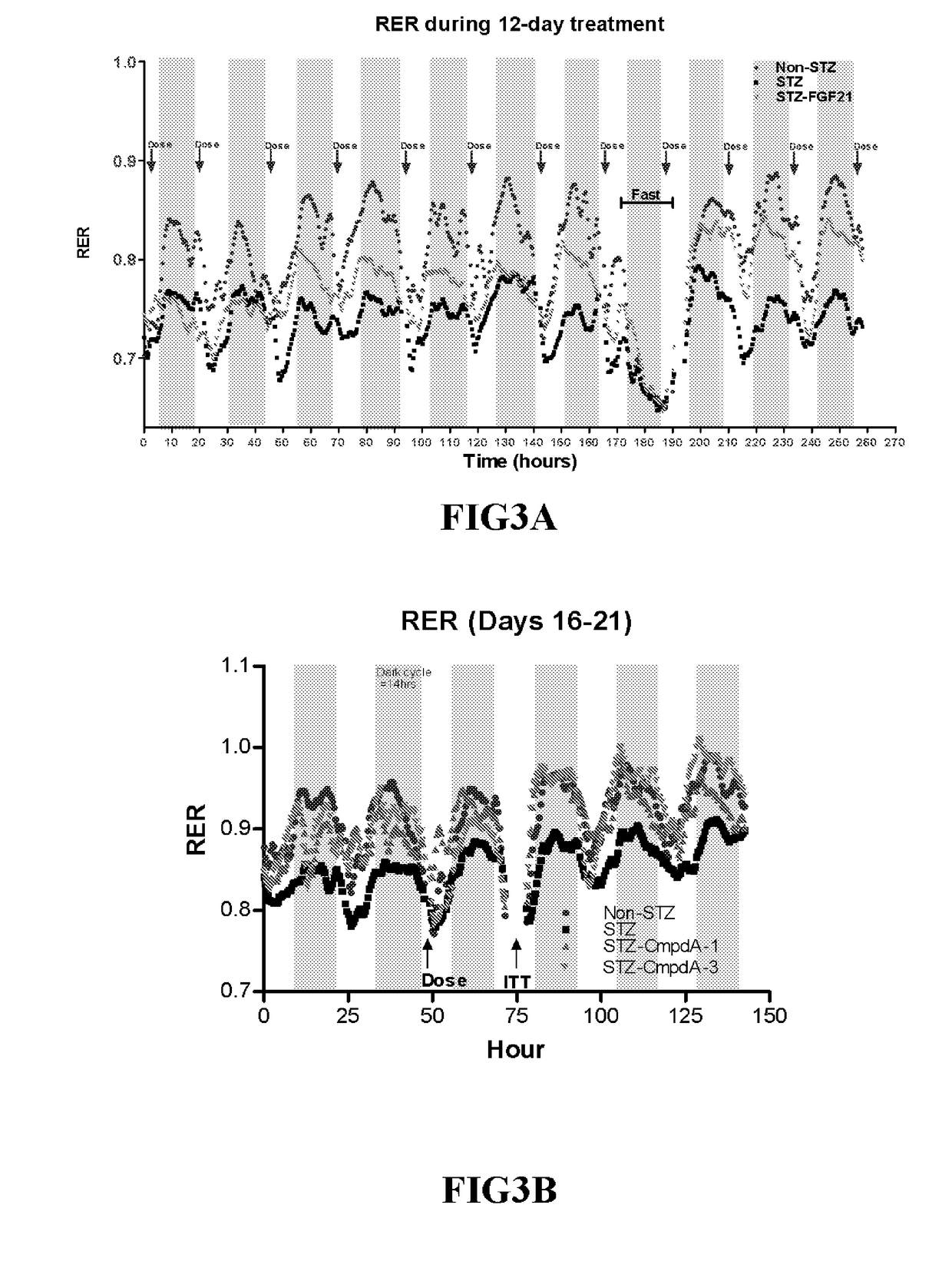
[0215]Human type 1 diabetes (T1D) exhibits high plasma glucose and low insulin levels due to the inability of pancreatic β-cell to produce and secrete insulin. As a consequence of a very low insulin level in circulation, patients with T1D have reduced utilization of carbohydrate and increased utilization of lipid, which leads to loss of body fat storage and ketosis.
[0216]High dosages of the β-cell toxin streptozocin (STZ) induce severe insulin deficiency and T1D with ketosis and hyperphagia. When high dose STZ is injected to adult animals, β-cell regeneration is diminished and animals remain in T1D condition.
[0217]Diabetes was induced in twenty-two-week old male C57BL mice via an intraperitoneal injection of STZ for 3 consecutive days at 70 mg / kg / day. When being fully diabetic (12-19 days from the first STZ injection), in two separate studies, the mice received either vehicle (PBS) or wild type FGF21 (3 mg / kg / day, subcut...
example 2
Type B Insulin Resistance: FGF21 Stimulates Glucose Uptake in Human Adipocytes in the Presence of Anti-Insulin Receptor Monoclonal Antibody
[0220]INSR knockout mice are not viable and typically die a few days after birth. While tissue specific knockout of INSR in mice is tolerated, the full spectrum of symptoms seen in patients with INSR mutation is not replicated in these animals. Thus, to further validate the clinical hypothesis that FGF21 can play a role in in the context of INSR inactivation, we simply measured the ability of FGF21 to promote glucose uptake in human adipocytes in the presence of a neutralizing anti-INSR antibody under conditions where insulin signaling is significantly impaired. This experiment effectively replicates the phenotype of Type-B insulin resistance (autoimmune insulin resistance), which is caused by development of neutralizing auto-antibodies to INSR.
[0221]Measurement of glucose uptake in differentiated human adipocytes is a widely used physiologically...
example 3
HIV HAART Induced Partial Liposystrophy
[0225]To study the effect of FGF21 in a model of lipodystrophy induced by HIV highly active antiretroviral therapy (HAART), 12-week-old C57BL mice (Taconic) were provided with PicoLab mouse diet #5058 (10% fat content) formulated by Research Diets with 0.1 or 0.2% HIV protease inhibitor Ritonavir. After 50 days of Ritonavir treatment, mice developed lipodystrophy and were divided into two subgroups, receiving either FGF21 V76 (5 mpk, s.c.) or PBS vehicle 2× / wk for 4 wks. Body weight, % body fat mass, plasma glucose, insulin and TG were measured during the study. Oral glucose tolerance test (OGTT) was assessed on treatment day 23. Liver lipid content was measured at the termination of the study.
[0226]Prior to V76 treatment, mice on diet mixed with Ritonavir developed a significant reduction in body weight gain (FIG. 9A) and fat content (FIG. 9B), as well as increased plasma triglyceride levels (FIG. 9C) relative to mice on control diet.
[0227]Bod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com