Transgenic animal model of mood disorders
a transgenic animal and mood disorder technology, applied in the field of psychiatric illness markers, can solve the problems of lack of conceptually novel antidepressants, high cost to the us economy, and significant morbidity and excess mortality, and achieve the effect of stimulating phosphorylation (activation)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Analysis of Brain Tissue Using Microarray Data Analysis
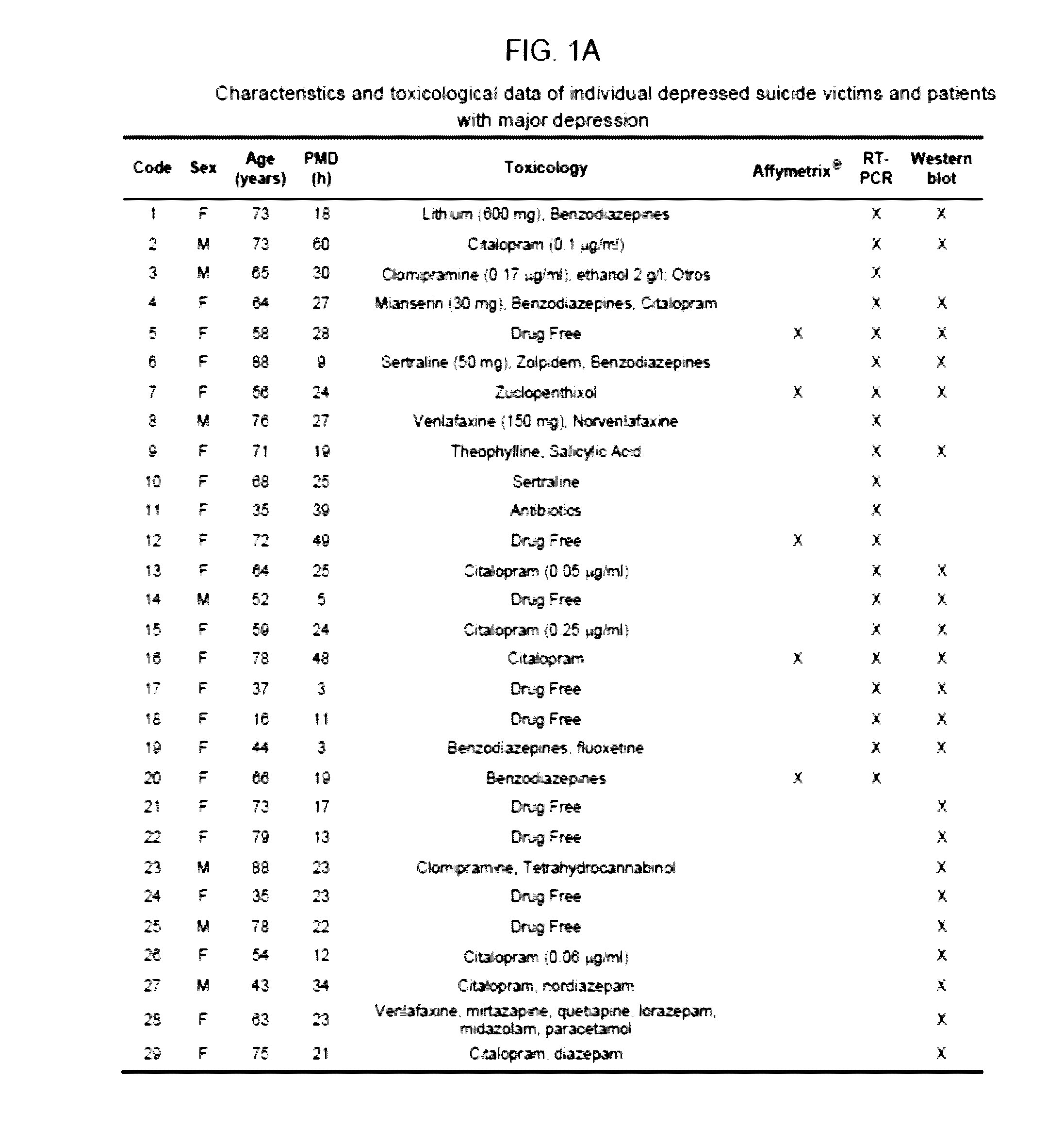
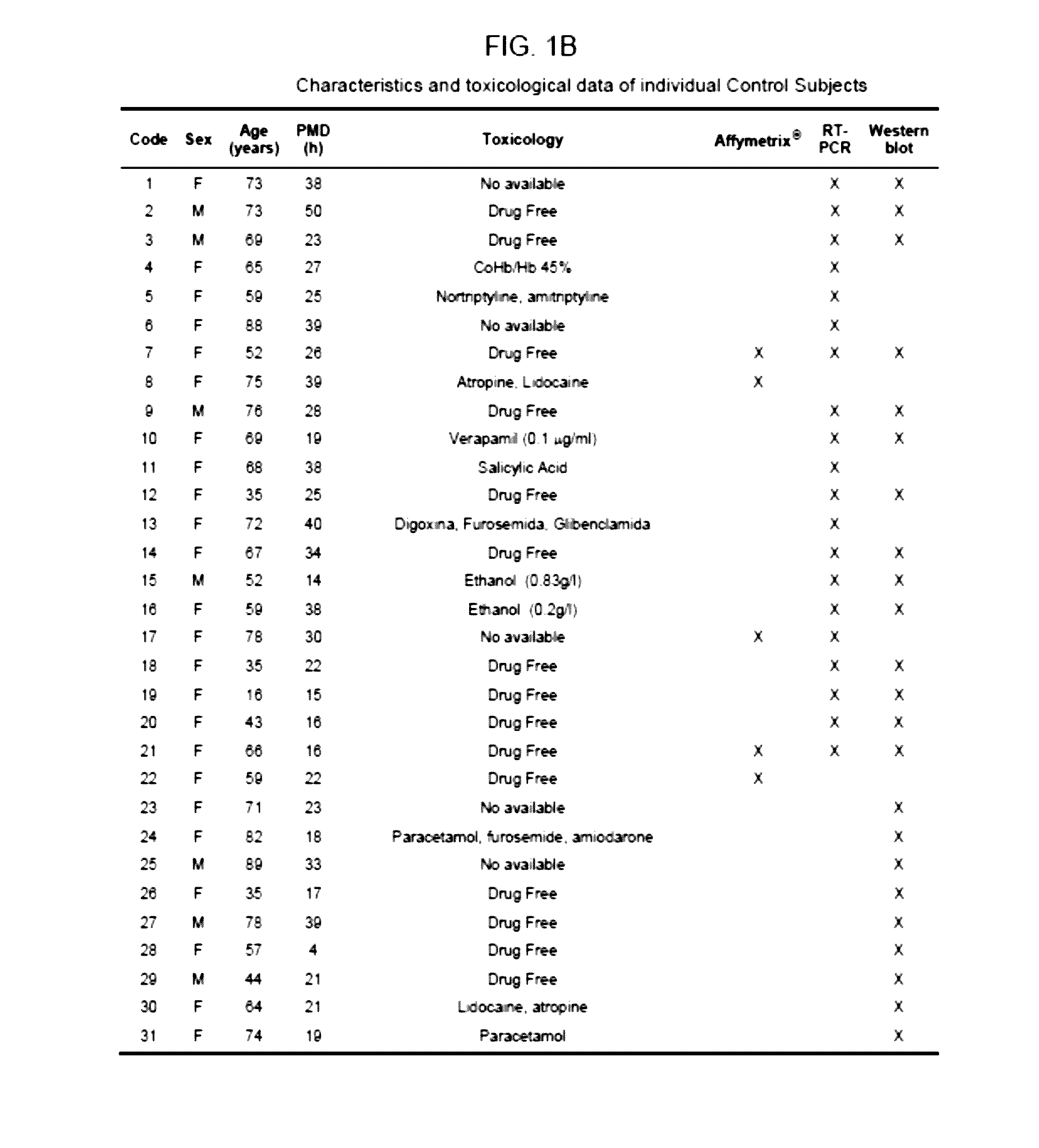
[0142]Samples of post-mortem prefrontal cortex (Broadmann's Area 9) were obtained at autopsies performed in the Forensic Anatomical Institute, Bilbao, Spain, and stored at −80° C. The study was developed in compliance with policies of research and ethical review boards for post-mortem brain studies (Basque Institute of Legal Medicine, Bilbao). Deaths recorded as suicide were subjected to retrospective careful searching for previous medical diagnosis and treatment using examiner's information and records of hospitals and mental health centres. Depressed victims were selected according to the following criteria: suicide by violent method, lifetime diagnosis of Major Depression made according to DSM-IV and DSM-IV-R (American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4rd edition revised. American Psychiatric Association, Washington DC, 2000), absence of psychotropic or illegal drugs. Matched con...
example 2
Determination of PTN as a Biological Marker for Depression
[0146]Microarray results were investigated using quantitative PCR. Eighty three genes were selected for quantitative PCR to validate their mRNA expression on a larger sample set, by using Low Density Arrays (Annex 2). Seven genes (RPL10, B2M, ENO2, ZFP207, ACTB, GAPDH and NEFH) were selected to be used as reference genes. Validation was carried out using Low Density Arrays (Applied Biosystems) in a total of 40 prefrontal cortex samples (20 case and 20 control samples). This tool allowed the simultaneous interrogation of all the chosen target genes with two replicates for each gene using TaqMan® technology. Briefly, a 384-well microfluidic card is loaded with the selected gene expression assays. Each card is composed of eight sample-loading ports, each connected to 48 reaction wells. One microgram of RNA from each sample was reversed transcribed using a high-capacity cDNA archive kit (Applied Biosystems) and random primers. 25...
example 3
Validation by Western Blotting of PTN as a Biological Marker for Depression
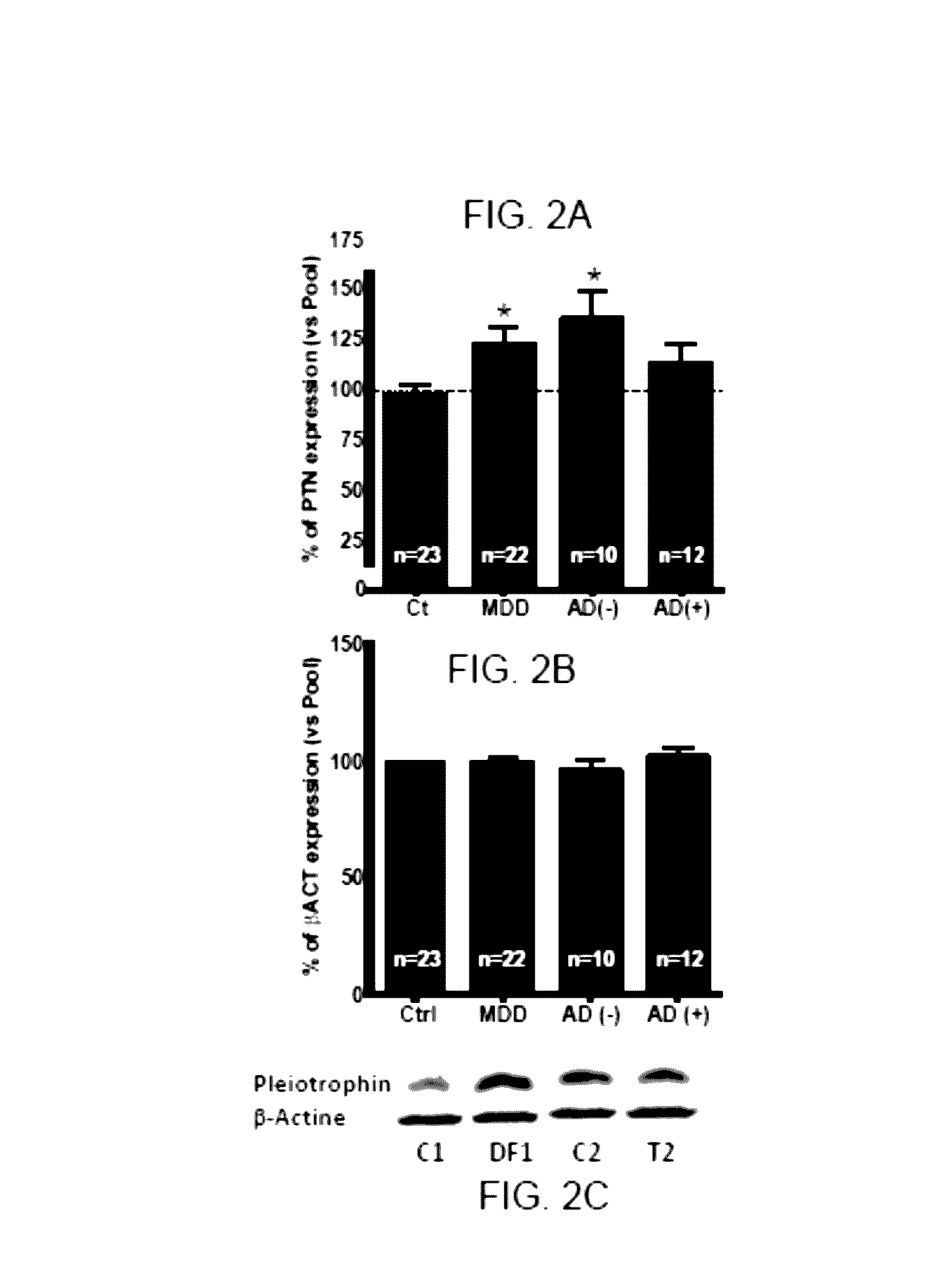
[0148]The levels of PTN protein in human cerebral prefrontal cortex samples (Brodmann area 9) from individuals diagnosed with depression who died by suicide (n=23) and who had (n=11) or who had not (n=12) received antidepressant treatment and control subjects with no history of psychiatric illness who died accidentally (n=23), were validated by Western blotting with an antibody that specifically recognises said protein. The samples from depressive patients were paired with control samples on the basis of sex, age and post mortem delay. Target protein was quantified in pairs of subject with depression and the respective matched control. The relative content of each protein was calculated (percent change) in relation to in-gel triplicate standards (100%, pool of controls samples). This quantification procedure was assessed 2-3 times in different gels, and the resulting mean value of the target protein was used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com