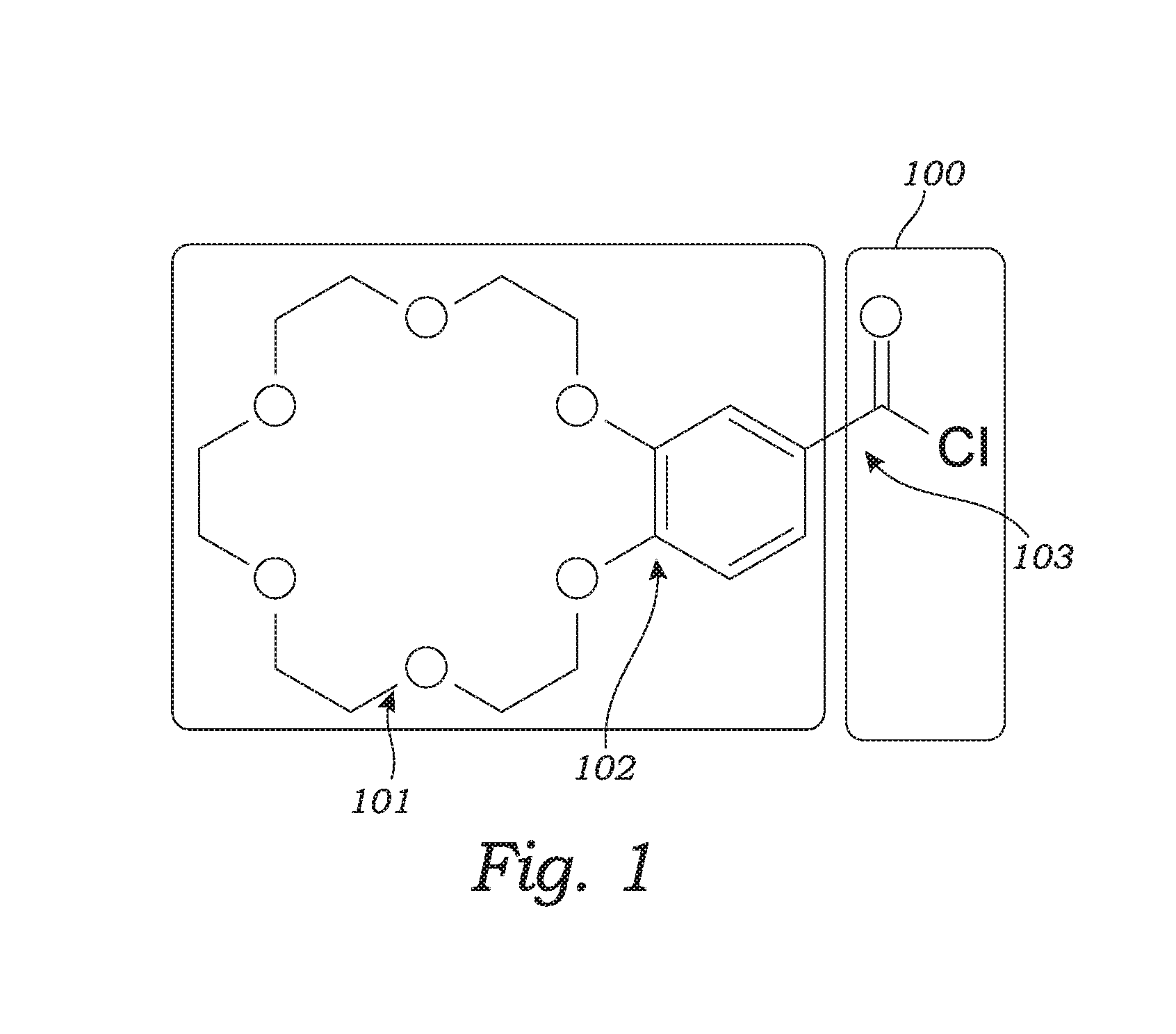
Solid Phase Extraction, Derivatization with Crown Ethers, and Mass Spectrometry, Methods, Reagents and Kits
a technology of derivatization and mass spectrometry, which is applied in the field of solid phase extraction, derivatization with crown ethers, mass spectrometry, methods, reagents and kits, can solve the problems of complex and cumbersome extraction procedures for quantification of certain analytes, and difficult detection of certain analytes, etc., to achieve similar sensitivity and accuracy, simple process, and easy configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Quantification of 1,25-Dihydroxy-Vitamin D in Plasma
[0207]Plasma samples were obtained from human patients' blood. Samples were drawn (plasma sodium heparin & EDTA) into pre-chilled Vacutainers. Vacutainers were inverted 5× and refrigerated until centrifuged. Plasma was separated in a refrigerated centrifuge (1000×g for 10 minutes) within 30 minutes of collection and then frozen immediately at −20° C. in plastic vials.
[0208]Plasma was thawed and diluted before use in solid phase extraction.
Standards
[0209]The blanks, calibration samples, and plasma samples were spiked with internal standards (e.g., D6-1,25(OH)2 vitamin D3 and / or D6-1,25(OH)2 vitamin D2).
[0210]Standard curves were generated with plasma solutions spiked with a known amount of 1,24-dihydroxy-vitamin D. The spiking solution was serially diluted before being added to the plasma taken from the same plasma sample.
Extraction
[0211]TRACE-N® (3 cc / 15 mg) columns were conditioned with 1.0 ml of methanol, followe...
example 2
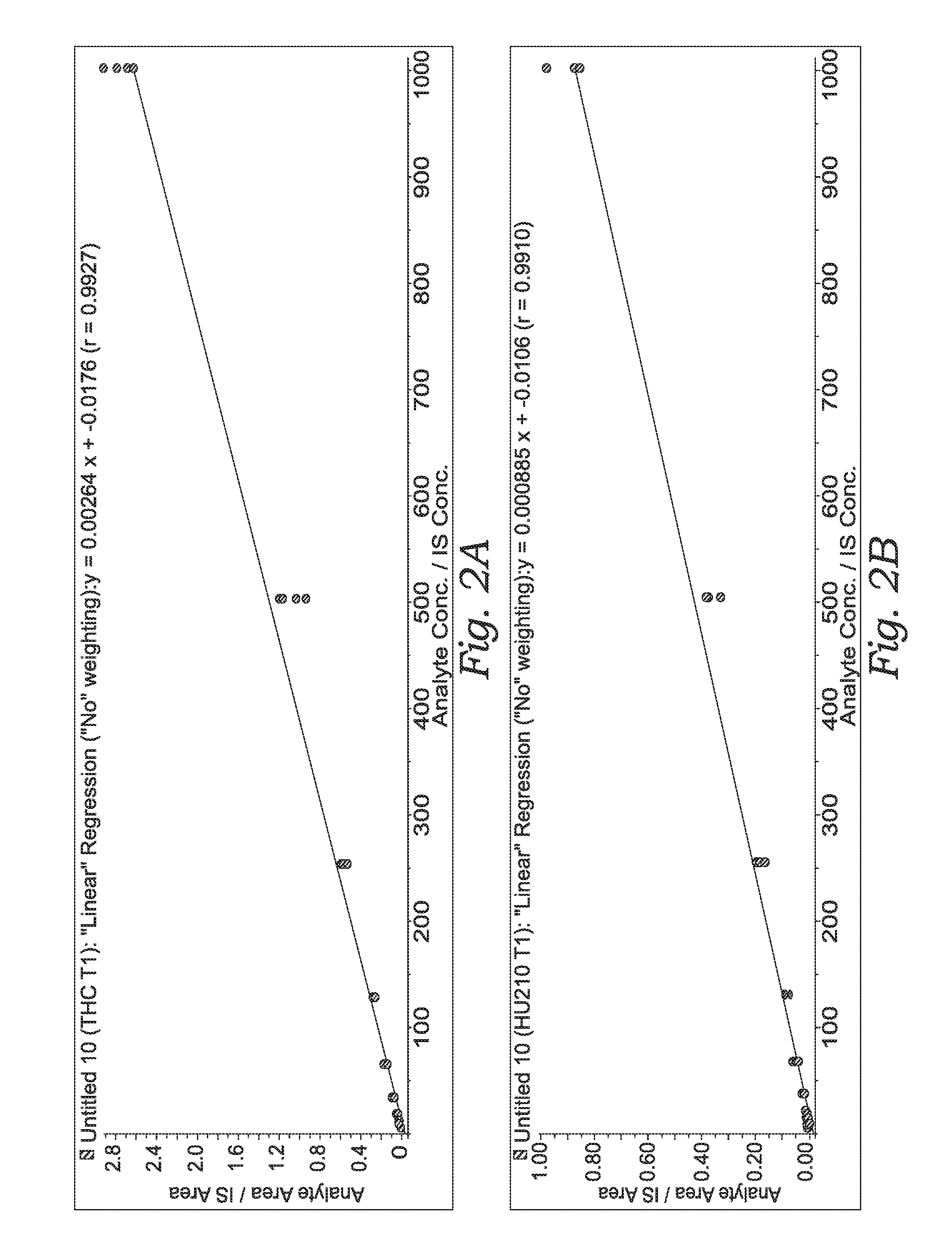
Quantification of THC or HU210 in Oral Fluid
[0222]Oral samples were taken from humans using the QUANTISAL™ (Immunalysis, California). Saliva was taken from patients using the collection device from QUANTISAL, and specimens were refrigerated and ultimately frozen. Saliva was thawed before use in the present quantification methods.
Standards
[0223]The blanks, calibration samples, and oral samples were spiked with internal standards (e.g., THC-d3).
[0224]Standard curves were generated with saliva solutions spiked with a known amount of THC or HU210 (a synthetic cannabinoid). The spiking solution was serially diluted before being added to the saliva taken from the same oral sample.
Extraction
[0225]TRACE-N® (3 cc / 15 mg) columns were conditioned with 0.5 ml of methanol, followed by 0.5 ml 0.1% HCl (6M) in water.
[0226]Then 300 uL of the oral fluid in QUANTISAL™ buffer (25% v / v). 10 μL internal standard was added and 600 uL of 100 mM pH6 phosphate buffer. The Sample / buffer mix ...
example 3
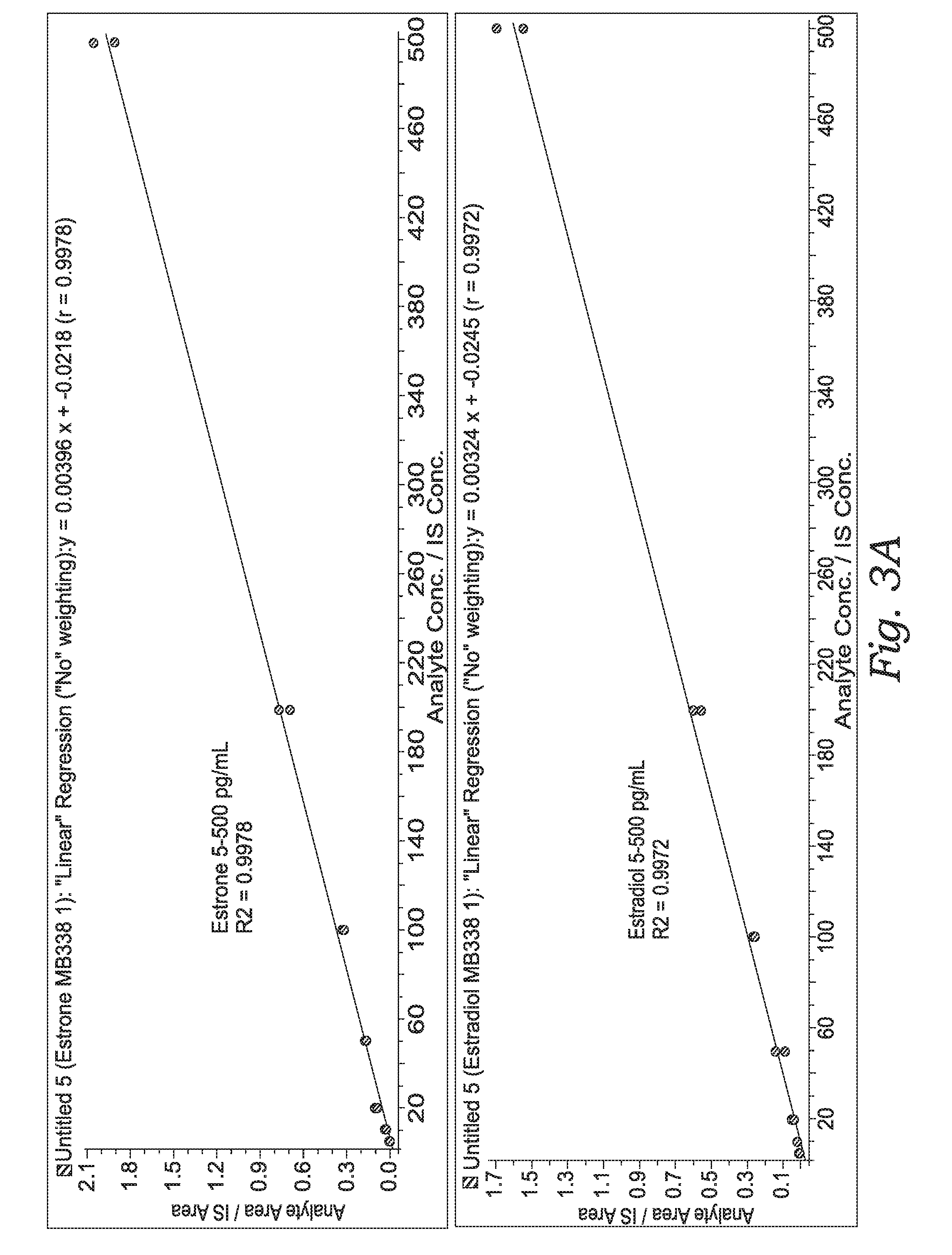
Quantification of Estradiol / Estriol / Estrone / 17β-Estradiol-2,3,4-13C3 in Plasma
[0235]Plasma samples were obtained from human patients' blood. Samples were drawn (plasma sodium heparin & EDTA) into pre-chilled Vacutainers. Vacutainers were inverted 5× and refrigerated until centrifuged. Plasma was separated in a refrigerated centrifuge (1000×g for 10 minutes) within 30 minutes of collection and then frozen immediately at −20° C. in plastic vials.
[0236]Plasma was thawed and diluted before use in solid phase extraction.
Standards
[0237]The blanks, calibration samples, and plasma samples were spiked with internal standards (e.g., 17β-estradiol-2,3,4-13C3).
[0238]Standard curves were generated with plasma solutions spiked with a known amount of estradiol or estrone. The spiking solution was serially diluted before being added to the plasma taken from the same plasma sample.
Extraction
[0239]MAESTRO® A (1 cc / 15 mg) columns were conditioned with 1.0 mL of methanol, followed by 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com