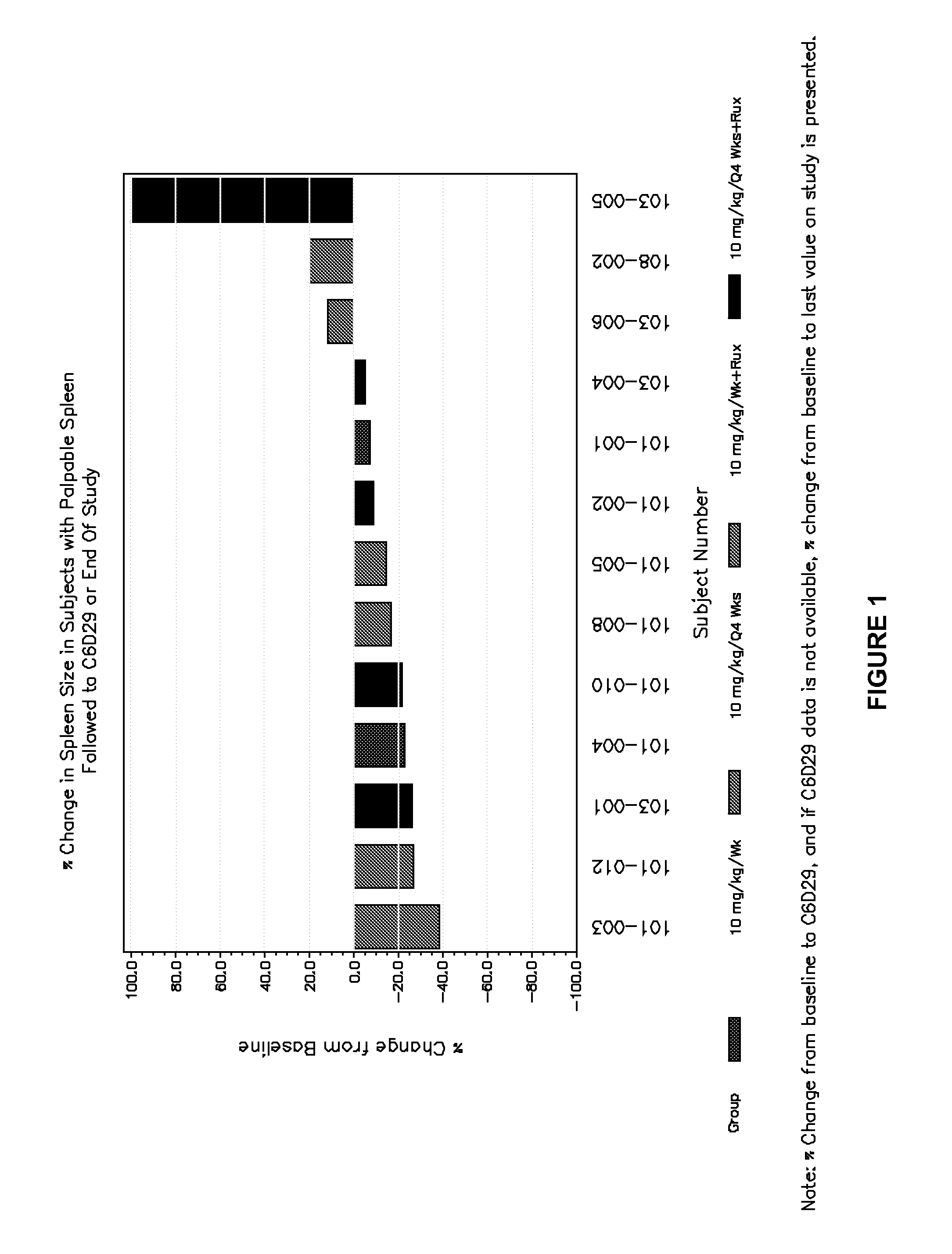
Methods for treating fibrotic cancers
a fibrotic cancer and treatment method technology, applied in the field of fibrotic cancer treatment methods, can solve the problems of poor clinical outcome, inadequate organ function, difficult treatment of fibrotic cancer, etc., and achieve the effect of improving efficacy and/or responsiveness to that agent, decreasing effectiveness, and improving efficacy and/or responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of Myelofibrosis with α2,3-Sialic Acid-Containing Sap Alone and in Combination with Ruxolitinib: Stage 1 Results at 12 Weeks
[0203]Recombinant human SAP, in this case the recombinant human SAP known as PRM-151, was administered to myelofibrosis (MF) patients, alone and in combination with ruxolitinib (RUX), to evaluate safety and efficacy in treating bone marrow fibrosis (BMF). Patients with Intermediate-1, Intermediate-2, or high risk MF with grade ≧2 BMF, either not receiving therapy or on a stable dose of RUX, were eligible for this study.
[0204]Twenty-seven patients were assigned to one of four cohorts based on administration of PRM-151 as a monotherapy or as part of a combination therapy. Cohort 1: i) patients who received no treatment for MF in at least two weeks, ii) were administered an initial loading dose of SAP at 10 mg / kg by intravenous infusion (˜1 hour infusion) on days 1, 3, and 5, and iii) thereafter were administered a dose of SAP every four weeks at 10 mg / k...
example 2
Treatment of Myelofibrosis with Prm-151 Alone and in Combination with Ruxolitinib: Stage 1 Results at 24 Weeks
[0209]Demographic data for the 27 patients enrolled in the four treatment arms of the study described above in Example 1 is summarized in Table 2 below. The patients enrolled in the study included those with primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (Post-PV MF) or post essential thrombocythemia myelofibrosis (Post-ET MF), classified as Intermediate-1, Intermediate-2, or high risk MF according to the Dynamic International Prognostic Scoring System (DIPSS). 15 of the 27 patients had been treated with a JAK inhibitor prior to the current study. Of the 27 patients who enrolled in the study and started treatment, 18 patients completed 24 weeks and evaluation was ongoing for 3 patients who had not reached the 24-week mark. Patient cohorts are as described above.
TABLE 2PRM-151 QWPRM-151 Q4WPRM-151 QW +PRM-151 Q4W + Demographic Info(Cohort 2)(Cohort 1)RUX (C...
example 3
Representative Individual Patient Data
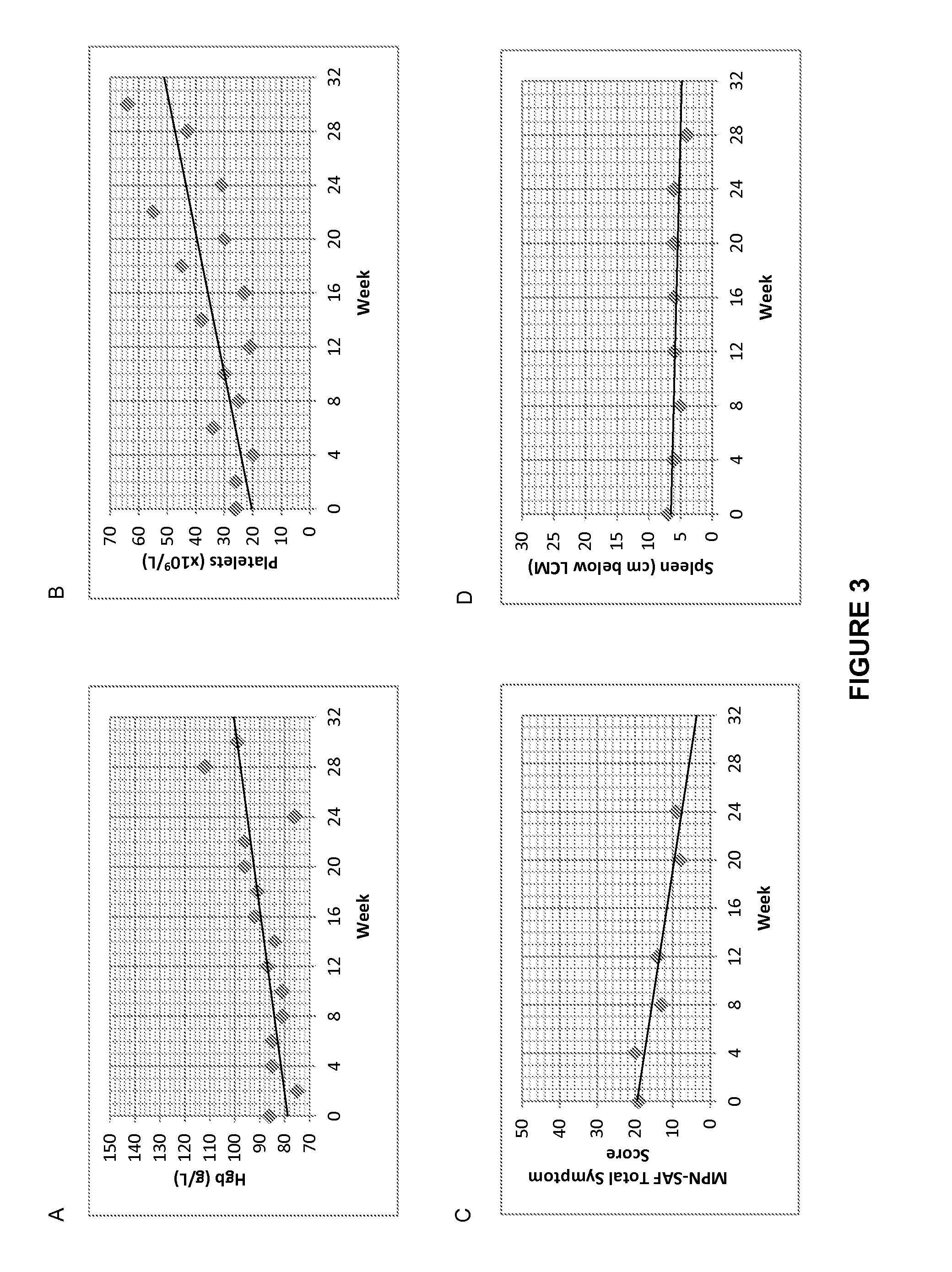
[0217]A representative example of response data from one responsive patient is shown here. The hemoglobin, platelet, MPN-SAF TSS, and spleen response trends to treatment with rhSAP over the course of 24 weeks for patient 101-005 are shown in FIGS. 3A-D. Patient 101-005 was treated with SAP every 4 weeks (Cohort 1). Patient characteristics are summarized in Table 4 below.
TABLE 4DIPSS Years since# priorTyperisk groupinitial diagnosistherapiesPMFIntermediate-273
[0218]As seen in FIG. 3, patient 101-005 showed an improvement in hemoglobin and platelet levels, a reduction in MPN-SAF TSS and a reduction in spleen size over the course of treatment. In addition, patient 101-005 showed a decrease in bone marrow fibrosis from Grade 2 to Grade 0 at 12 weeks (FIG. 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com