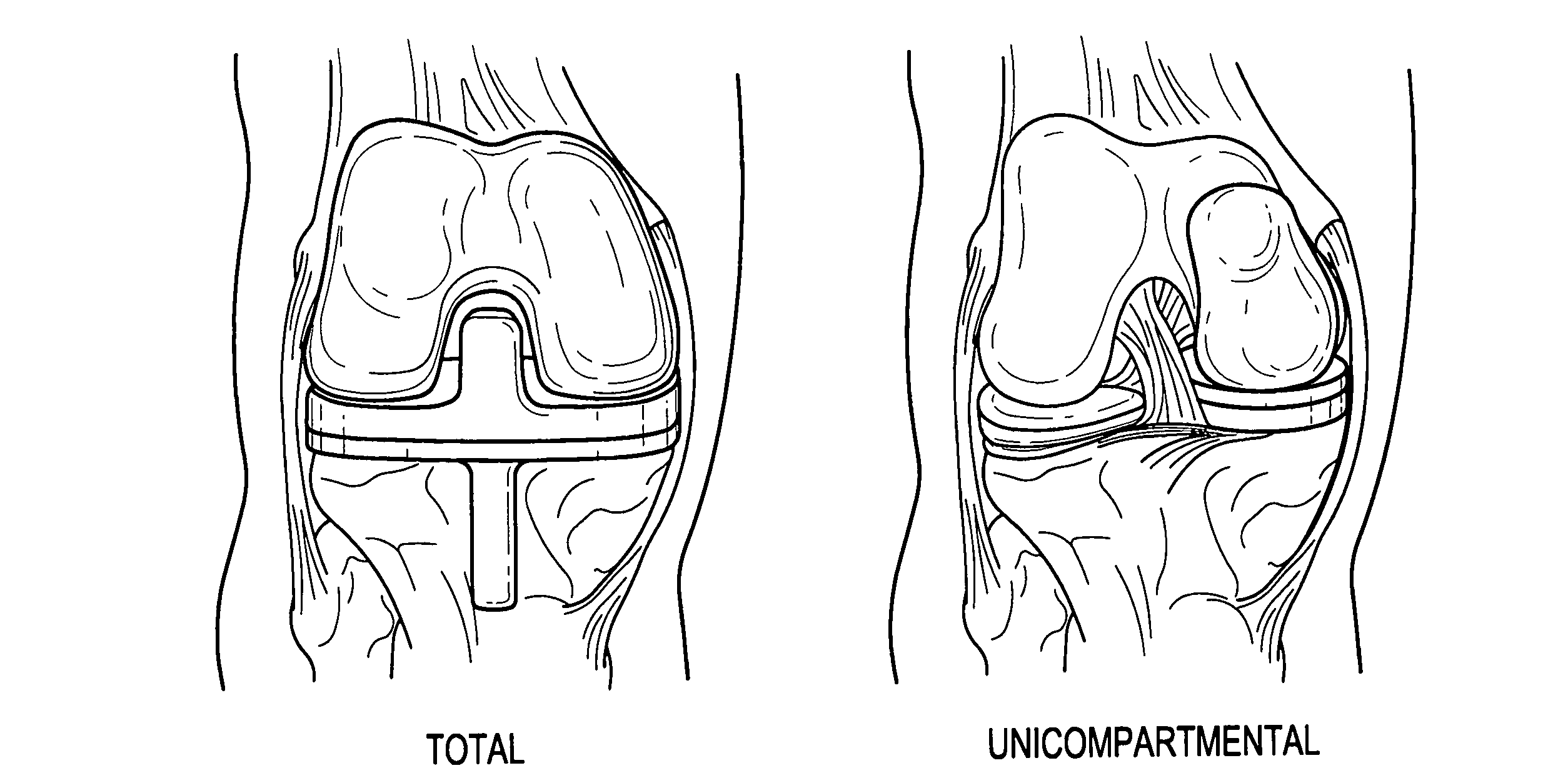
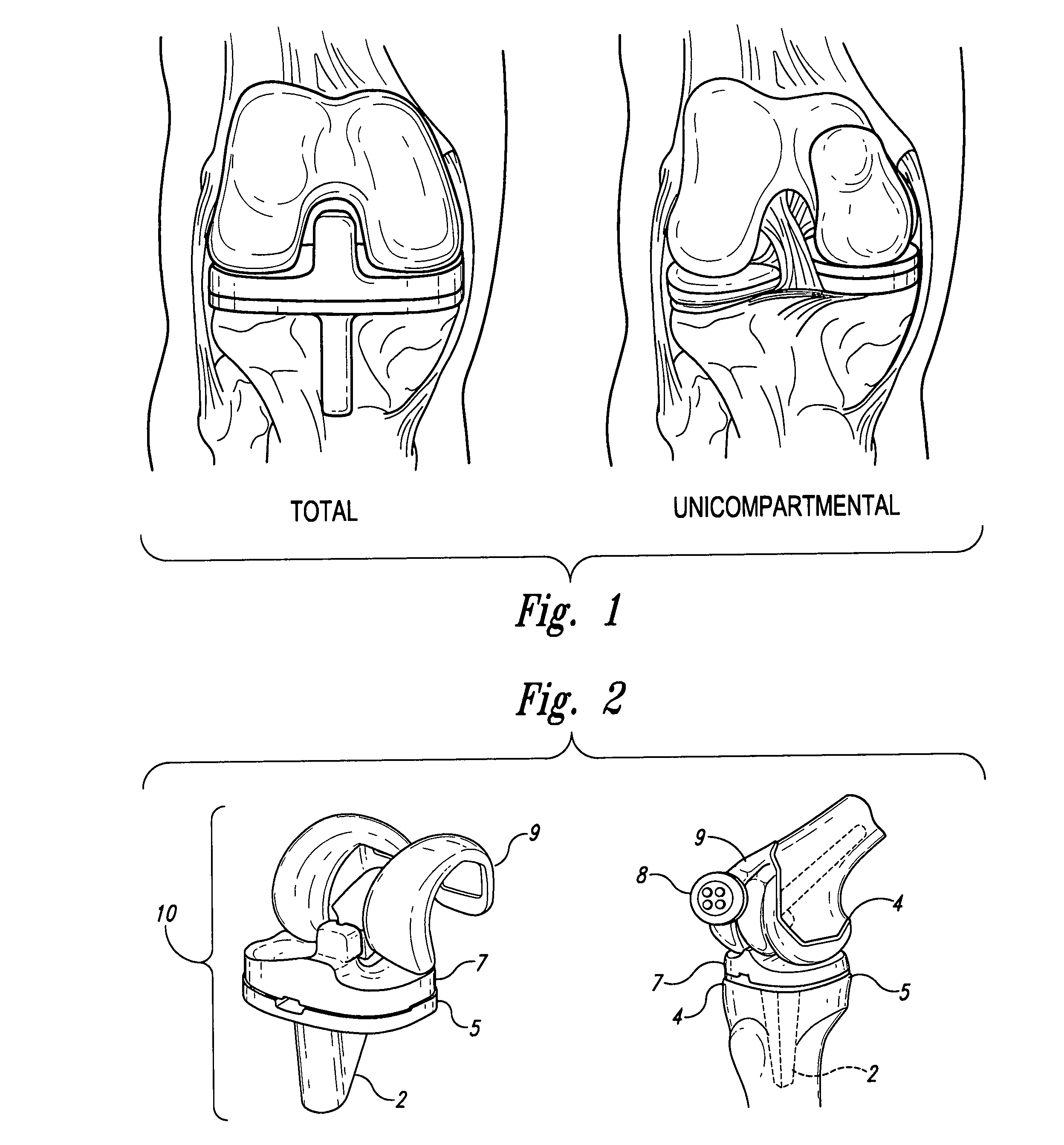
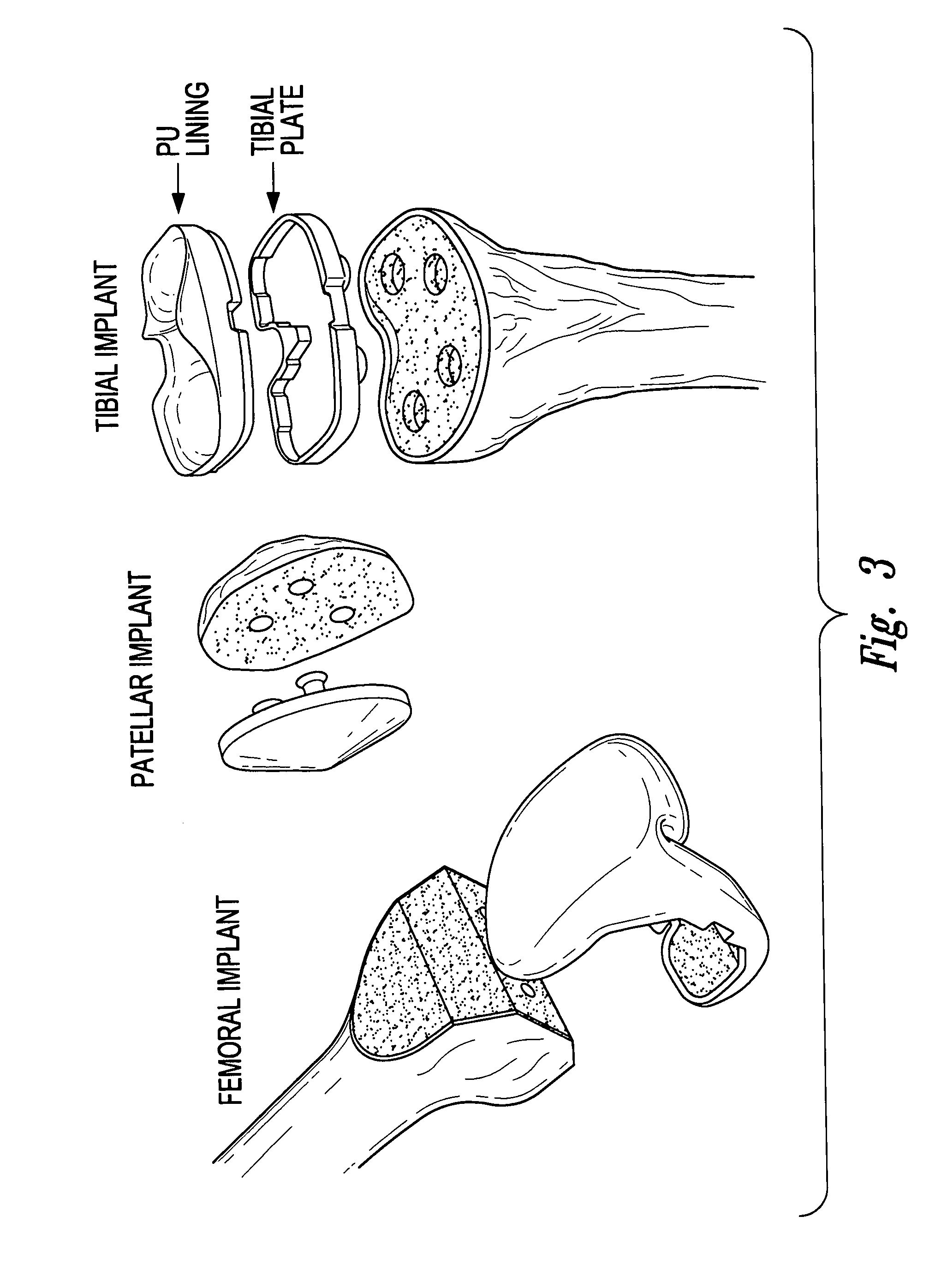
Devices, systems and methods for monitoring knee replacements
a technology for knee replacements and devices, applied in the field of knee replacements, can solve the problems of knee joint improper operation, patient discomfort, and various complications, and achieve the effect of being easily incorporated into bone cement or
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0143]2) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the tibial component.
[0144]3) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the patellar prosthesis.
[0145]4) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a sensor on the femoral component.
[0146]5) The knee replacement prosthesis according to any one of embodiments 1 to 4 wherein said sensor is selected from the group consisting of accelerometers, pressure sensors, contact sensors, position sensors, chemical microsensors, tissue metabolic sensors, mechanical stress sensors and temperature sensors.
embodiment 5
[0147]6) The knee replacement prosthesis wherein said accelerometer detects acceleration, tilt, vibration, shock and or rotation.
[0148]7) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes contact sensors positioned on the femoral component.
[0149]8) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a plurality of contact sensors positioned on the patellar component.
[0150]9) The knee replacement prosthesis of embodiment 1 wherein the plurality of sensors includes a plurality of contact sensors positioned on the tibial component.
[0151]10) A medical device, comprising a femoral component of a knee replacement prosthesis and a plurality of sensors coupled to said femoral component.
[0152]11) A medical device, comprising a patellar prosthesis of a knee replacement prosthesis and a plurality of sensors coupled to said patellar prosthesis.
[0153]12) A medical device, comprising a tibial component of a knee replac...
embodiment 14
[0156]15) The medical device wherein said accelerometer detects acceleration, tilt, vibration, shock and or rotation.
[0157]16) The knee replacement prosthesis according to any one of embodiments 1 to 9 or medical device according to any one of embodiments 10 to 15 further comprising:
[0158]an electronic processor positioned upon and / or inside at least one of the tibial component, patellar prosthesis and / or the femoral component that is electrically coupled to sensors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com