Therapeutic agent for meibomian gland dysfunction
a meibomian gland and therapeutic agent technology, applied in the field of meibomian gland dysfunction therapy agent, can solve the problems of lowering the quality of life of many patients, not knowing the effective treatment method of mgd, and not being clear for those skilled in the ar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Test Using Complete Freund's Adjuvant Administered Rabbit
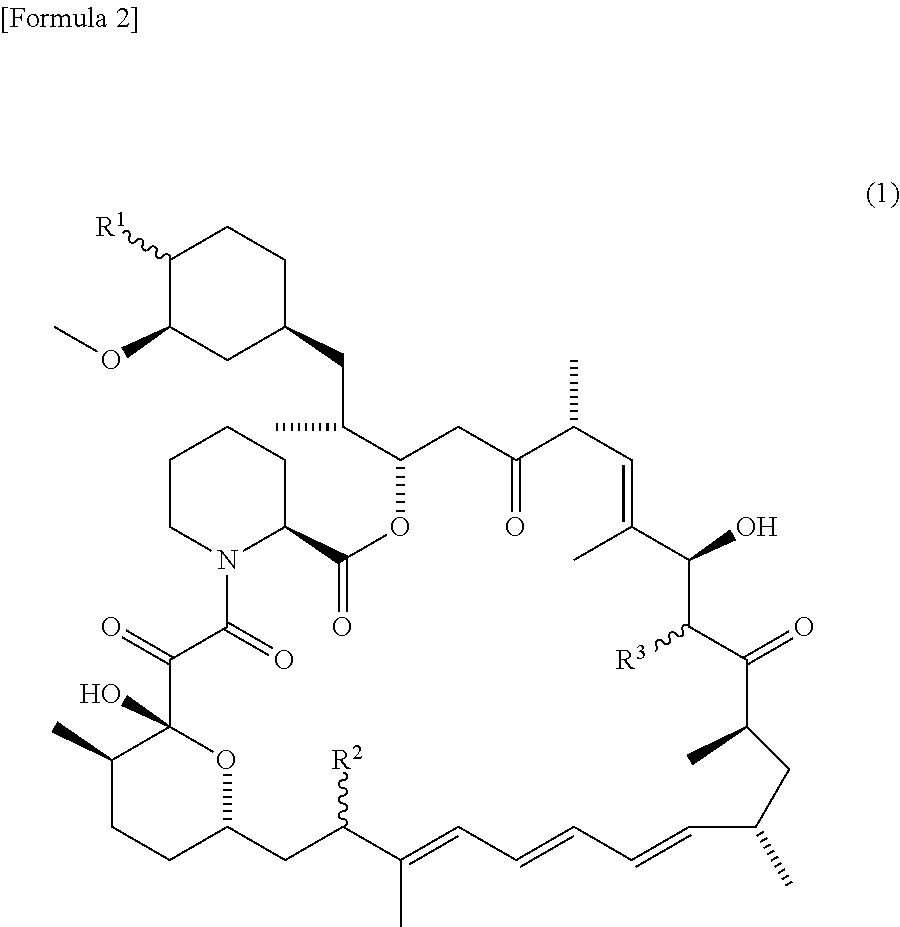
[0102]In a complete Freund's adjuvant administered rabbit, telangiectasia around the meibomian gland orifices, and obstruction at the meibomian gland orifices, which are similar to the signs / findings of MGD can be recognized. Effects of sirolimus which was a representative example of the present compound on the telangiectasia and obstruction were investigated (see JP 2014-024835A).
[0103](Sample Preparation)
[0104]0.1% (w / v) sirolimus suspension: it was prepared by suspending in 0.01% (w / v) hydroxypropylmethyl cellulose. Incidentally, with regard to sirolimus, that purchased from LKT Laboratories, Inc., (Catalog number: R0161) was used (which is the same in the following Examples).
[0105](Test Method)
[0106]10 μL of the complete Freund's adjuvant was administered to the right upper eyelid (3 portions) of about 2 kg of male Japanese white rabbits, respectively. After 4 days from provocation, around the meibomian gland orifices of t...
preparation examples
[0125]The drugs of the present invention are more specifically explained by referring to Preparation examples, but the present invention is not limited by these Preparation examples alone.
prescription example 1
Eye Drop (0.1% (w / v))
[0126]
In 100 mlSirolimus0.1gMedium chain fatty acid triglyceride (MCT)7.5gBenzalkonium chloride0.2gTyloxapol1.2gPoloxamer 1881gGlycerin22.5gSterile purified waterq.s.
[0127]To sterile purified water are added sirolimus and the above-mentioned components other than these, and the mixture is thoroughly mixed to prepare an eye drop. Characteristics of the present eye drop is an emulsion. By changing the formulation amount of the sirolimus, eye drops with the concentration of the sirolimus of 0.05% (w / v), 0.5% (w / v) or 1% (w / v) can be prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com