Nuclease resistant polynucleotides and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
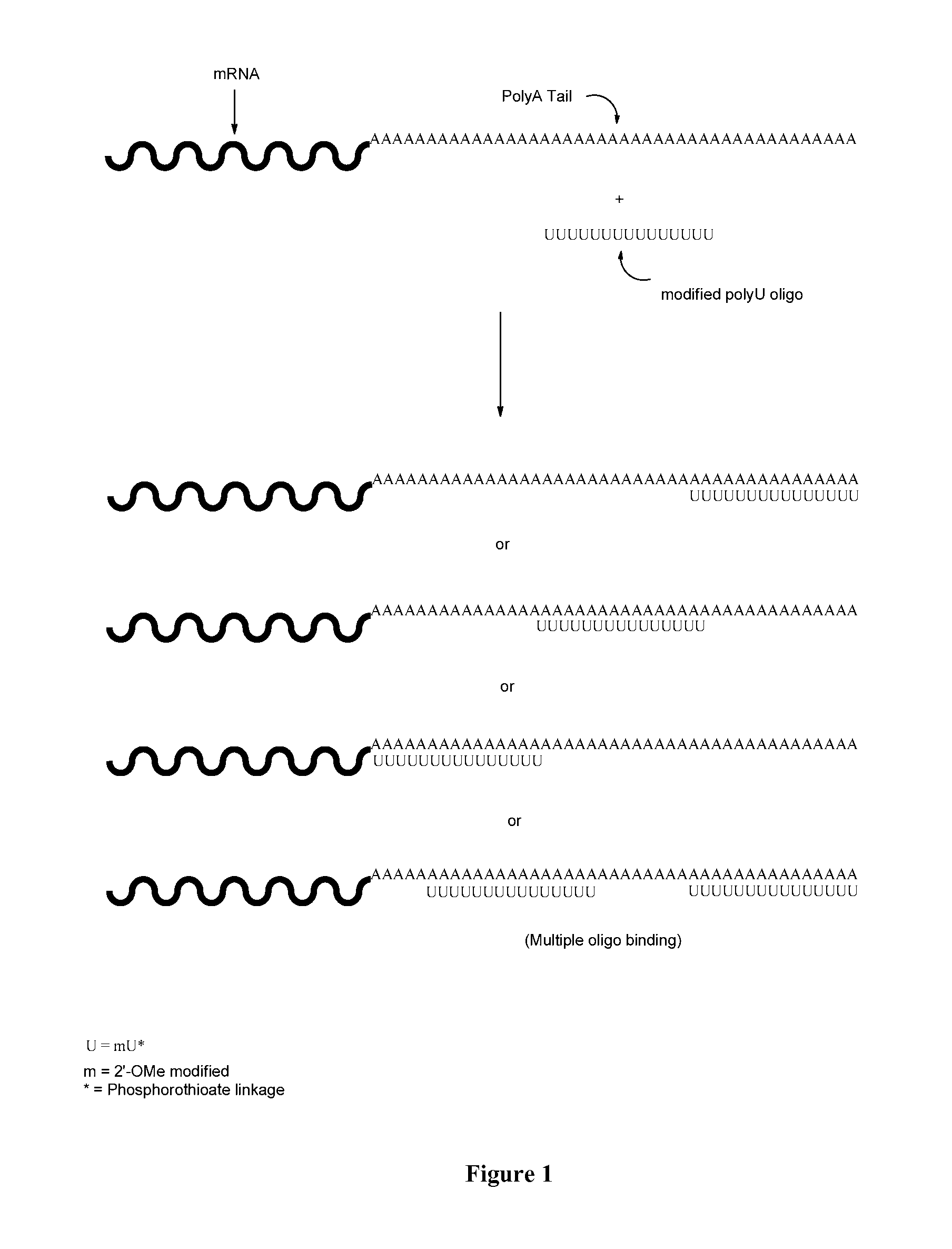
[0103]The present example illustrates the ability of stabilizing oligonucleotides of the present invention to enhance the production of protein when co-administered with non-denatured in vitro transcribed mRNA. Without wishing to be bound by any theory, it is contemplated that the stabilizing oligonucleotides modulate the nuclease resistance and increases the translational efficiency of mRNA polynucleotide transcripts.
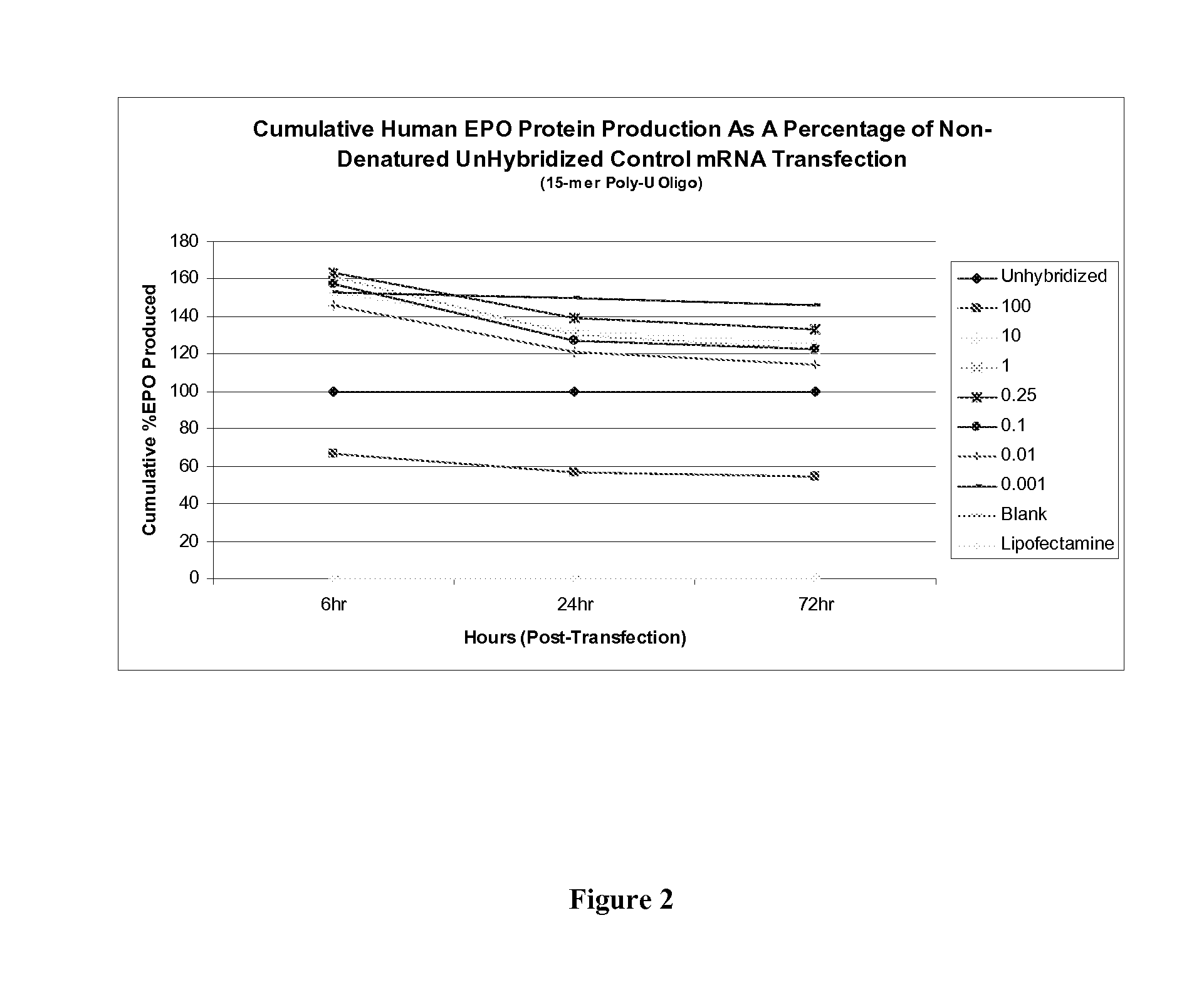
[0104]To perform the instant studies, a 15-mer (2′O-Me-uracil) stabilizing oligonucleotide having a phosphorothioate backbone (MW=4965.8 g / mol) was prepared and which was designed to be complementary to the poly-A tail of an mRNA polynucleotide (MW=299605 g / mol) encoding human erythropoietin (EPO) protein. The EPO mRNA transcript was contacted with the stabilizing oligonucleotide at 0.001:1, 0.01:1, 0.1:1, 0.25:1, 1:1, 10:1 and 100:1 parts stabilizing oligonucleotide to mRNA polynucleotide. The resultant stabilized mRNA transcripts (designated “0.001”, “0.01”, “0.1”, “...
example 2
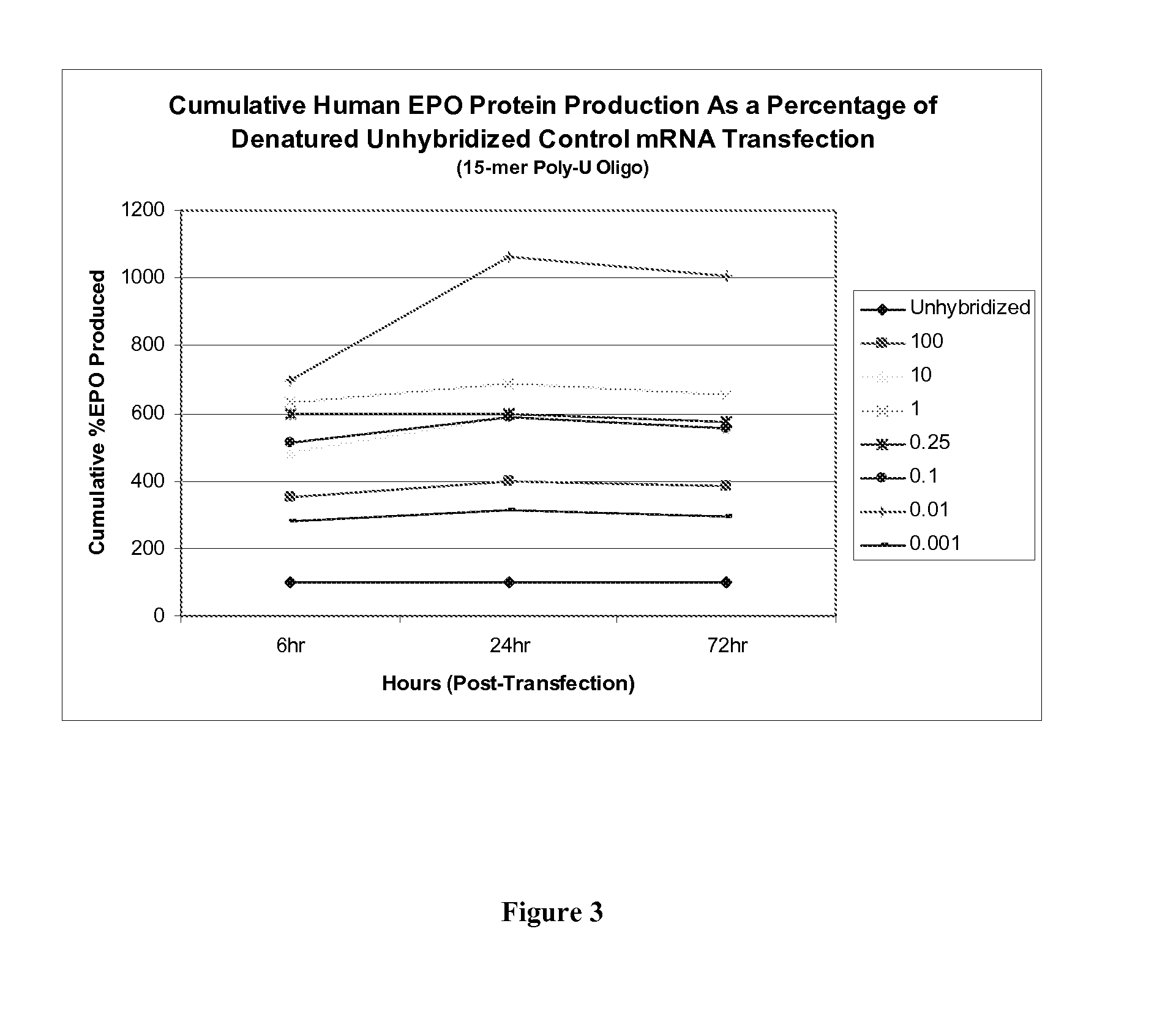
[0106]The present example further illustrates the ability of the stabilizing oligonucleotides of the present invention to enhance the protein production by first hybridizing to a denatured single-stranded mRNA to form a stabilized mRNA before administering into cells for protein production
[0107]As described in Example 1 above, a 15-mer (2′O-Me-uracil) stabilizing oligonucleotide having a phosphorothioate backbone was prepared and which was designed to be complementary to the poly-A tail of an mRNA polynucleotide encoding human erythropoietin (EPO) protein. The EPO mRNA transcript was first denatured at 65° C. for 10 minutes, and then contacted with the stabilizing oligonucleotide at 0.001:1, 0.01:1, 0.1:1, 0.25:1, 1:1, 10:1 and 100:1 parts stabilizing oligonucleotide to mRNA polynucleotide. The resultant stabilized mRNA transcripts (designated “0.001”, “0.01”, “0.1”, “0.25”, “1”, “10” or “100”) or the untreated, denatured EPO polynucleotide control transcript (designated “Unhybridiz...
example 3
[0110]The instant study was performed to investigate optimal length of the stabilizing oligonucleotides of the present invention.
[0111]A 30-mer (2′O-Me-uracil) stabilizing oligonucleotide having a phosphorothioate backbone was prepared and which was designed to be complementary to the poly-A tail of an mRNA polynucleotide encoding human erythropoietin (EPO) protein. A non-denatured EPO mRNA transcript was contacted with the stabilizing oligonucleotide at 0.001:1, 0.01:1, 0.1:1, 0.25:1, 0.5:1, 1:1 and 2:1 parts stabilizing oligonucleotide to mRNA polynucleotide. The resultant stabilized mRNA transcripts (designated “0.001”, “0.01”, “0.1”, “0.25”, “0.5”, “1” or “2”) or the untreated, non-denatured EPO polynucleotide control transcript (designated “Unhybridized”) were then transiently transfected into 293T cells. The cumulative amounts of EPO protein produced and expressed by the transfected 293T cells were then measured at 24, 48, 72 and 96 hour intervals.
[0112]As illustrated in FIG. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com