Sustained release formulations of lorazepam
a formulation and sustained release technology, applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of limited stability reduced relative bioavailability of sustained release tablets, and limited auc, so as to achieve favorable steady-state pharmacokinetic profiles and prolong absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044]Sustained release lorazepam beads were made having the following nominal composition.
Reference toUnit QualityCompositionComponentStandardsFunctionmg / Unita% w / wLorazepamUSPActive ingredient2.00 3.0Hypromellose K100 USPRelease control0.67 1.0premium LVagentStarch pregelatinizedNFBinder6.67 10.0Microcrystalline NFFiller57.33 86.0cellulose specialPurified water—bTotal66.67100.0a= Equivalent weight of core beads to obtain a 2 mg dose of Lorazepam.b= Removed during processing.USP = United States Pharmacopeia;NF = National Formulary
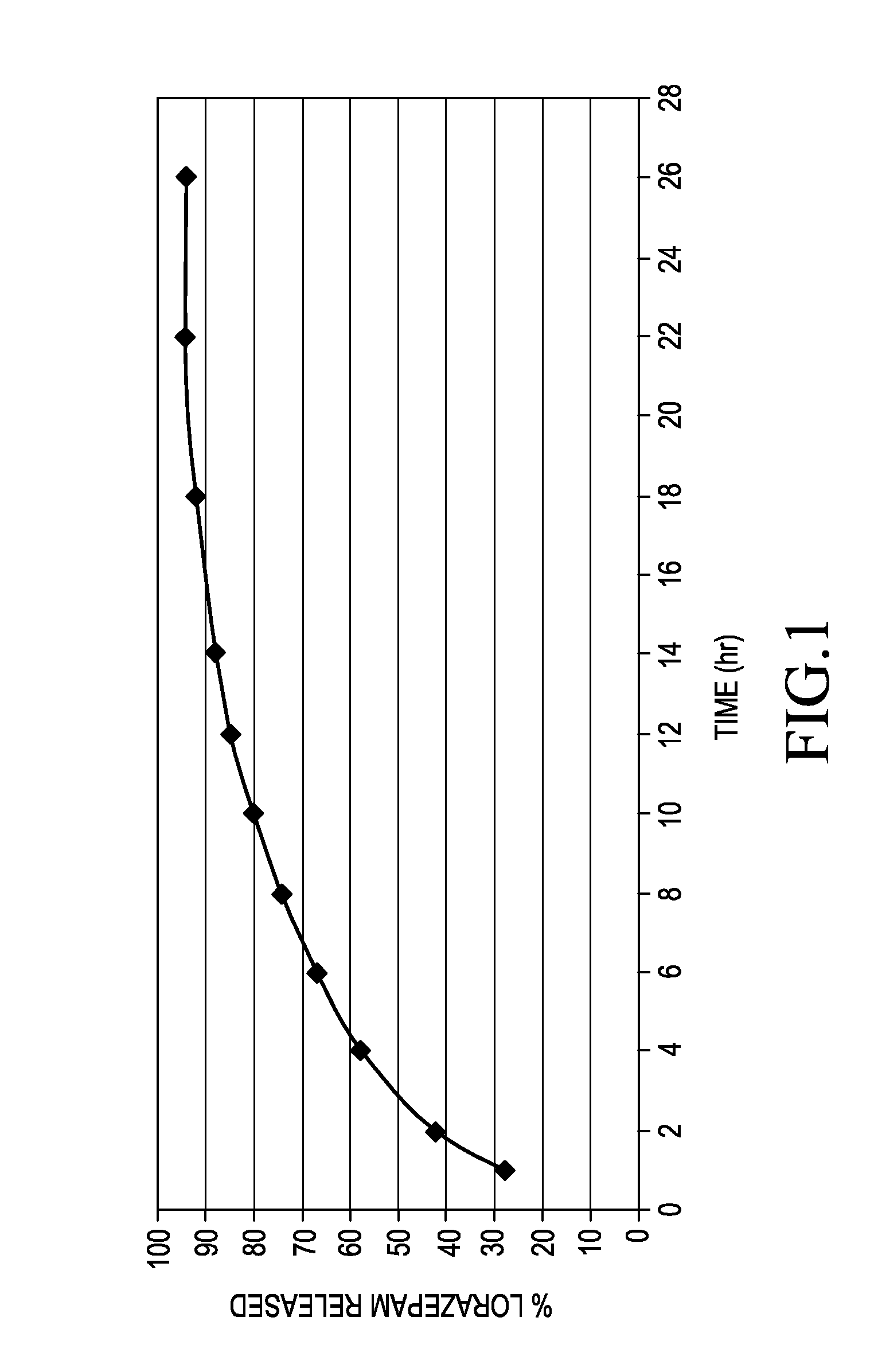
[0045]The beads were made by screening (30 mesh) the lorazepam, HPMC, and starch and mixing for 5 minute increments. The MCC was screened (30 mesh) and subsequently added with 10 minutes of mixing. The dry mixture was granulated with the addition of water, extruded, and spheronized. The beads were dried to a residual moisture content of less than 3% w / w and fraction screened between 40 mesh and 25 mesh. Samples of the beads were subjected to a two media i...
example 2
[0046]Delayed sustained release lorazepam beads were made by using the above sustained release lorazepam beads and applying an enteric coating. The enteric coating was designed to release at pH 7 or higher and specifically was intended to permit free release at pH 7.4. The resulting delayed sustained release lorazepam beads have the following nominal composition.
Reference toUnit QualityCompositionComponentStandardsFunctionmg / Unita% w / wExample 1 beads USPActive ingredient66.7 82.11(core)Eudragit FS 30DDelayed release12.1 14.92control agentPlasACRYL T20Plasticizer1.21 1.49Colloidial silicon USPAnti-tacking 1.20 1.48dioxide (optional)agentPurified water—bTotal81.21100.0a = Equivalent weight of coated beads to obtain a 2 mg dose of Lorazepam. Coating level is for a 20% weight gain.b = Removed during processing.USP = United States Pharmacopeia.
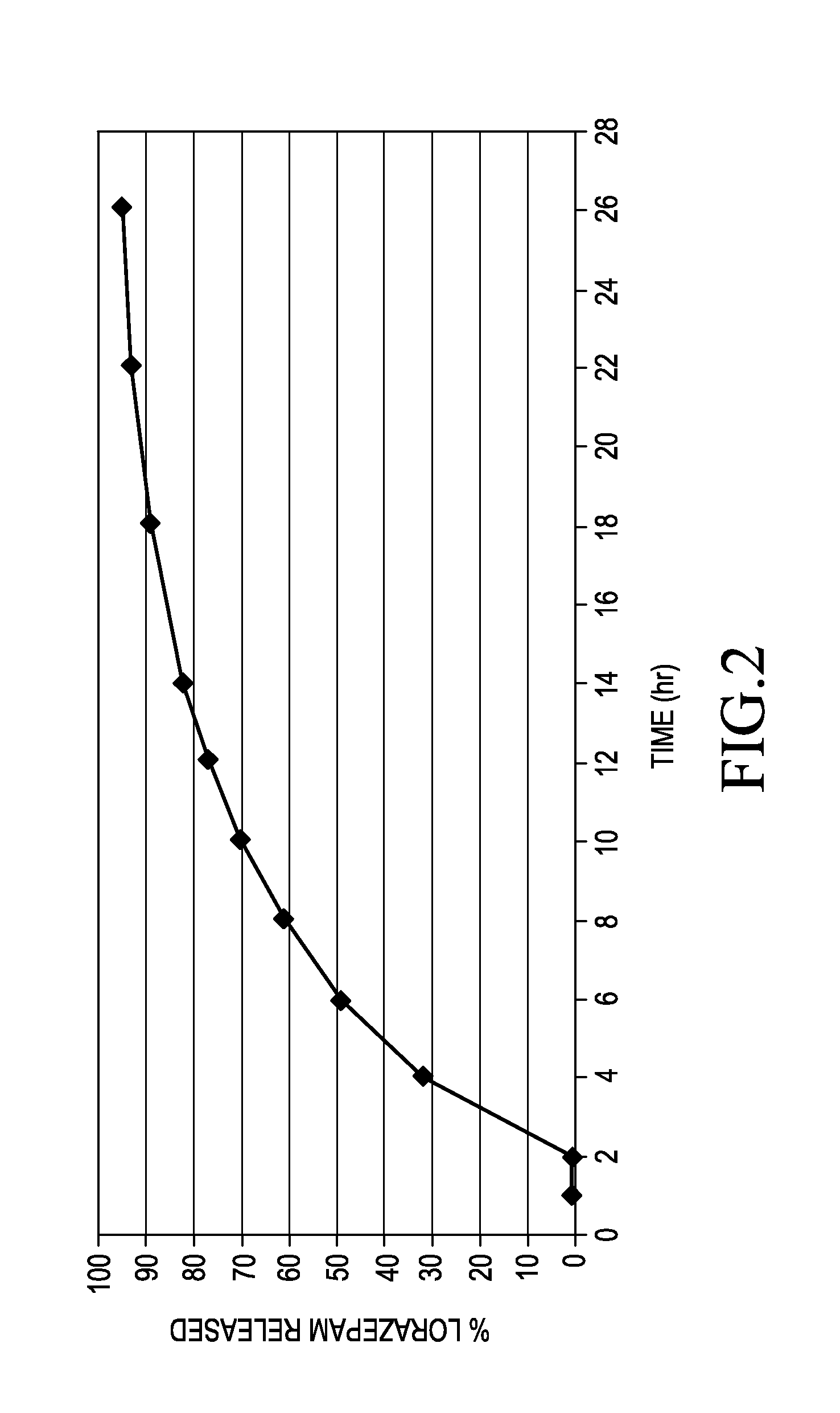
[0047]The delayed sustained release lorazepam beads were made by coating the beads obtained according to example 1. The Eudragit, plasticizer, a...
example 3
[0051]A pharmaceutical composition containing sustained release lorazepam beads and delayed sustained release lorazepam beads was formed by filling a capsule with the beads of Examples 1 and 2. A hard gelatin capsule was filled with the following nominal amounts of ingredients to form a 2 mg lorazepam oral dosage form.
Reference toUnit QualityCompositionComponentStandards Functionmg / Unit% w / wExample 1 beads33.3345.09%Example 2 beads40.6054.91%Hard gelatin capsule shellCapsule1 unit—Total73.93100.0 a = 2 mg Lorazepam dose. The contribution is 50% from the Example 1 beads and 50% from the Example 2 beads.
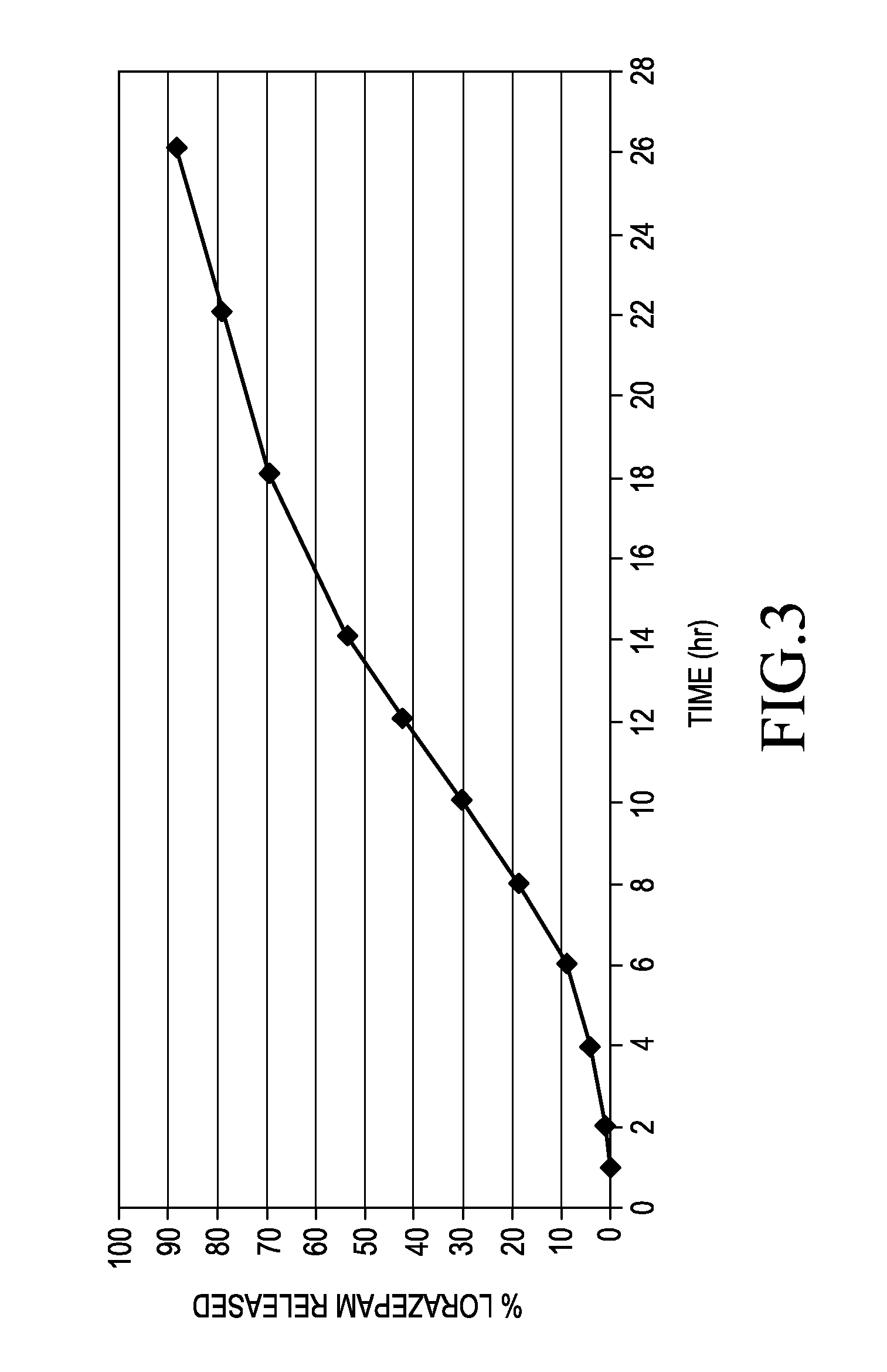
[0052]The nominal 2 mg lorazepam capsules were evaluated in a single dose pharmacokinetic study involving 24 subjects. The dose was administered under fasting conditions. The study lasted for 120 hours. The study average showed that lorazepam was being absorbed beyond 30 hours. The calculated steady state values show that therapeutic concentrations of lorazepam are maintained over a 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com