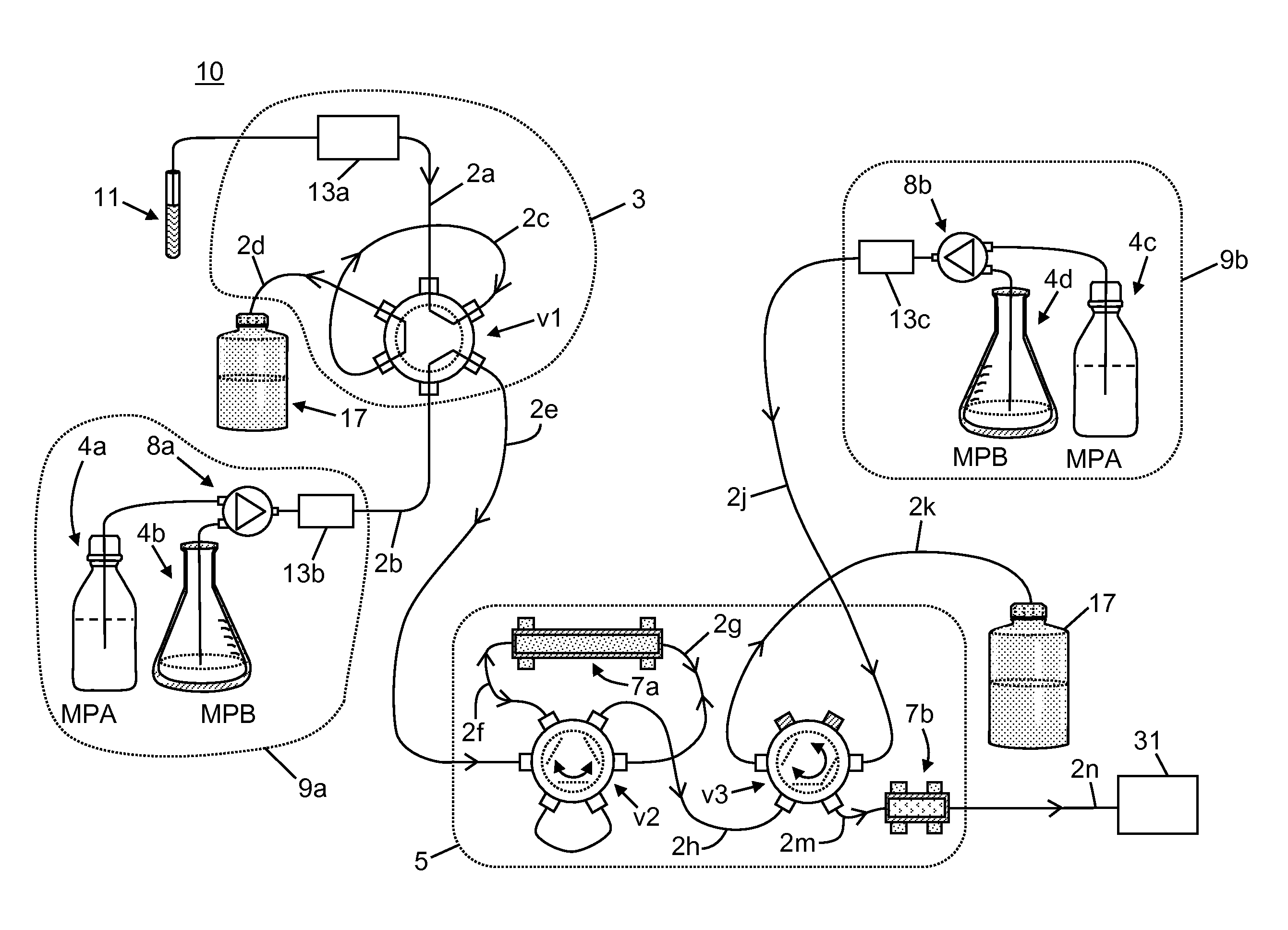
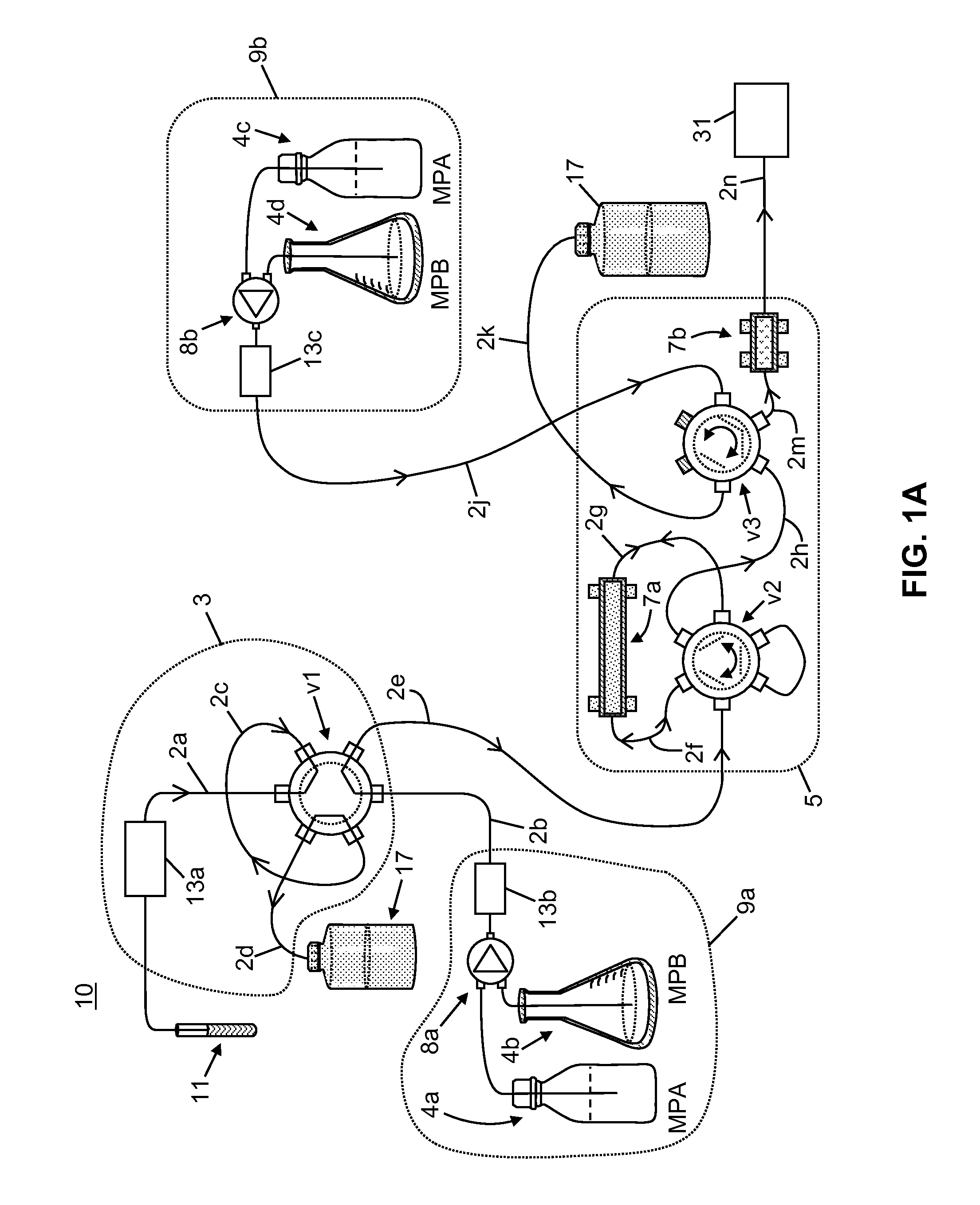
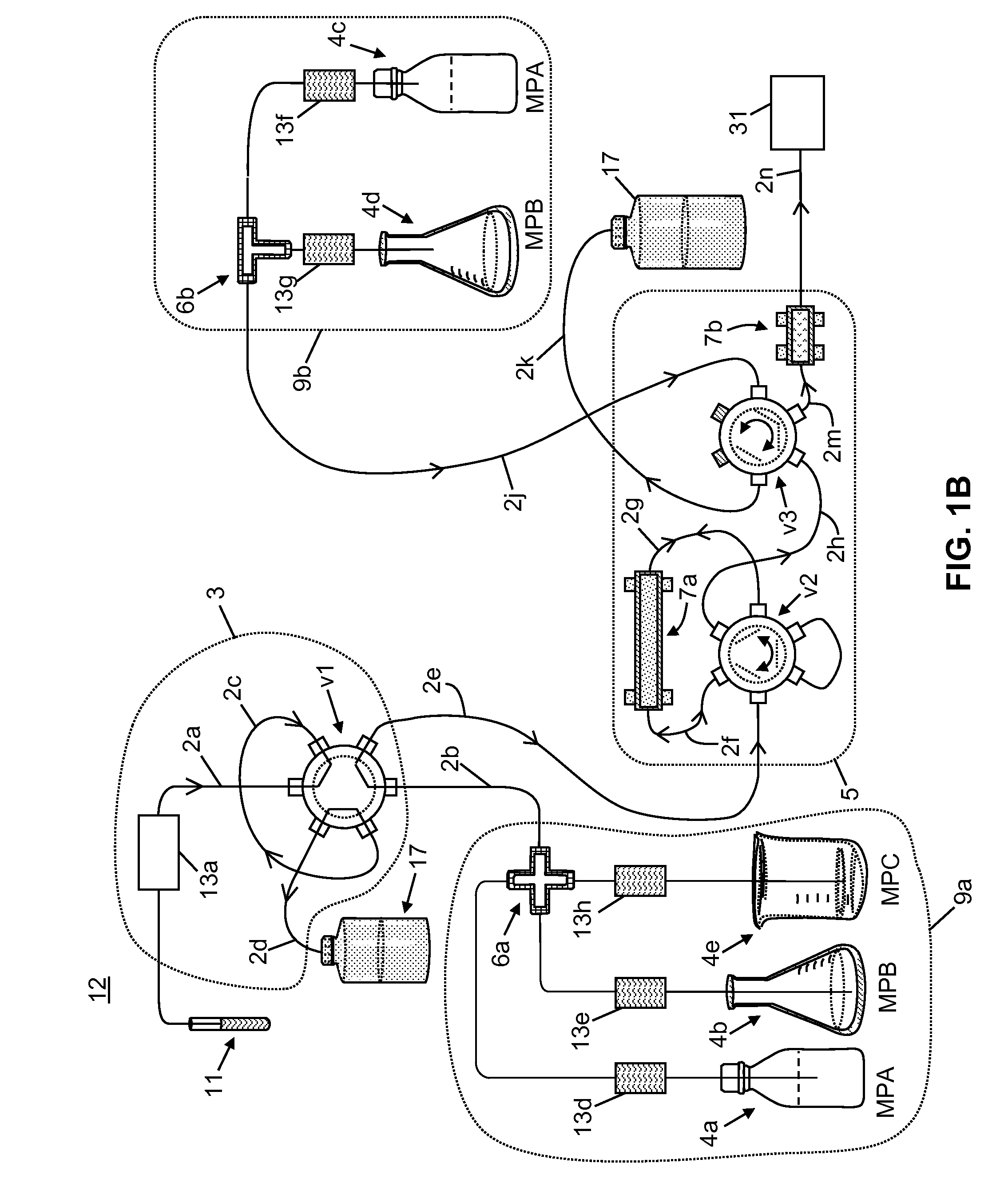
Apparatus for Improved Immunosuppressant Drug Monitoring
a technology for immunosuppressant drugs and apparatus, which is applied in the field of immunosuppressant drugs in biological samples, can solve the problems of insufficient chromatographic runs to remove all bound phospholipids from the column, and achieve the effect of reducing the number of chromatographic runs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Materials and Methods
[0058]The following chemicals, used as solvents and additives, were obtained from Fisher Scientific™ of Waltham, Mass., USA: acetonitrile (LC-MS grade), acetone (HPLC grade), methanol (LC-MS grade), isopropanol (HPLC grade), water (LC-MS grade), ammonium hydroxide, formic acid, and zinc sulfate heptahydrate, 99%. A stock solution of tacrolimus-13CD2, used as an internal standard, was obtained from Toronto Research Chemicals. A stock solution of D12-cyclosporin A, used as a second internal standard, was obtained from Alsachim.
[0059]A neat tacrolimus internal standard solution was prepared at a concentration of 1000 ng / mL by mixing 20 μL of the stock solution in 20 mL of methanol. A neat cyclosporin A internal standard solution was prepared at a concentration of 10000 ng / mL by mix 200 μL of the stock solution in 20 mL of methanol. The internal standard solutions were stored at a temperature of −20° C. or lower until needed. An aqueous zinc sulfate solution for lys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com