Efficient stabilizer in controlling self accelerated decomposition temperature of peroxycarboxylic acid compositions with mineral acids
a technology of peroxycarboxylic acid and self-accelerated decomposition temperature, which is applied in the direction of anhydride/acid/halide active ingredients, biocide, disinfection, etc., can solve the problems of limiting commercial opportunities, posing an extreme explosion risk, and commercial package siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0187]Self-Accelerating Decomposition Test. As used herein, SADT refers to the lowest temperature at which self-accelerating decomposition may occur with the peracid composition. In some embodiments, SADT refers to the lowest temperature at which self-accelerating decomposition may occur under the commercial packaging, storage, transportation and / or use condition(s). SADT can be estimated, calculated, predicted and / or measured by any suitable methods. For example, SADT can be estimated, or measured directly by one of 3 methods (H1, H2 and H4) recommended by the UN Committee for the Transportation of Dangerous Goods in “Recommendations on the Transport of Dangerous Goods, Model Regulations” (Rev.17) ST / SG / AC.10 / 1 / Rev.17. For example, the methodology disclosed in Malow and Wehrstedt, J. Hazard Mater., 120(1-3):21-4 (2005) can be used, which is herein incorporated by reference in its entirety.
[0188]The full test protocol used in this Example is available at “Recommendations on the Tran...
example 2
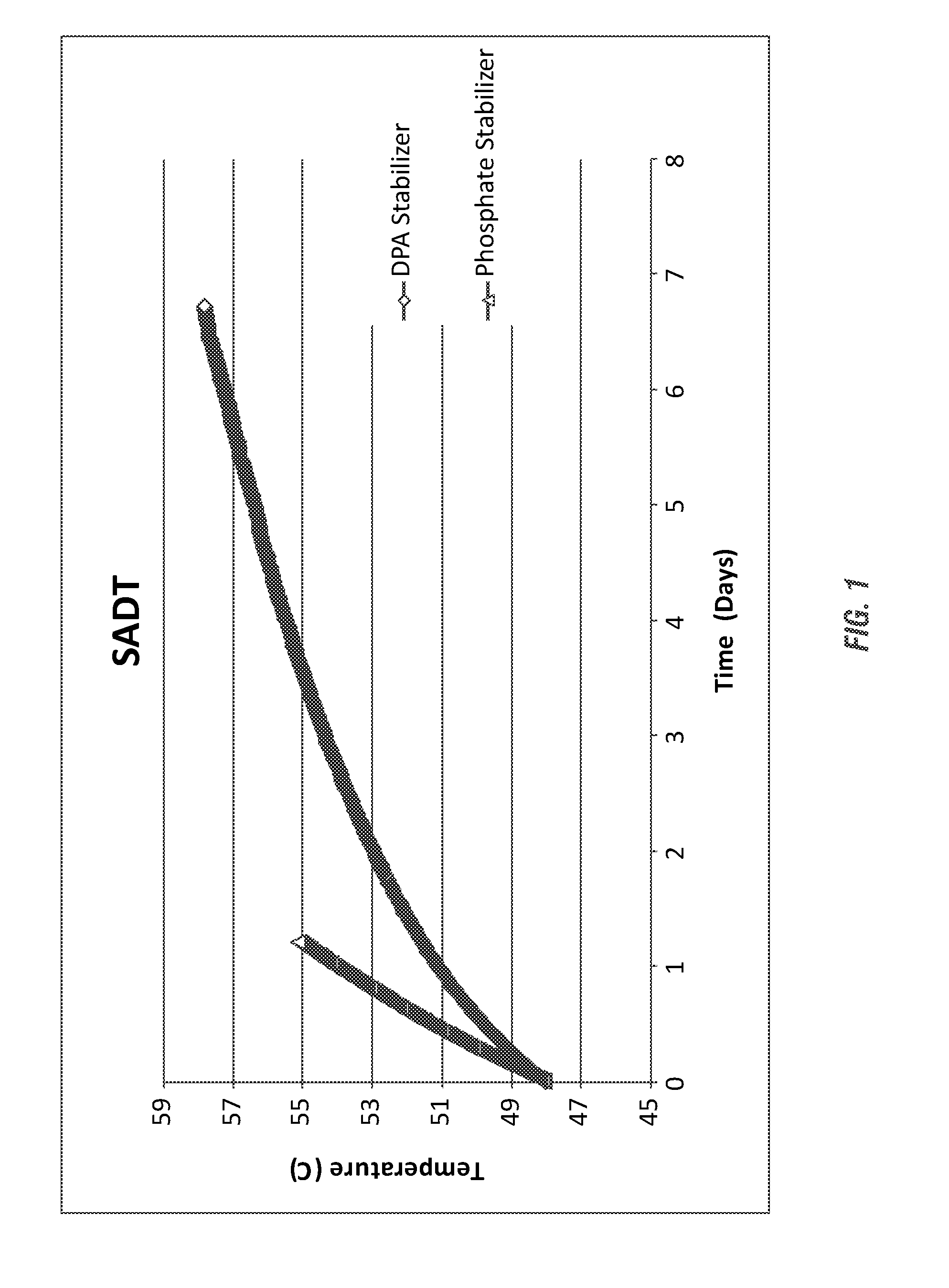
[0192]The methods of Example 1 were further employed to analyze the SADT of a fatty peracid, a peroxyoctanoic acid compositions having an even further increased acidity. While the formulae were identical aside from their stabilizers, the results again illustrate an advantage in DPA stabilization in a high acid environment that extends to fatty peracids such as peroxy octanoic acid.
TABLE 3Wt %CompositionHEDP FormulaDPA FormulaDPA0.0 0.05HEDP (60%) 2.540.0Octanoic acid 1-10 1-10Sodium octane sulfonate (40%)10-3010-30H2O2 (35%)10-5010-50H2SO4 (96%)14.1414.14Deionized Water20-5020-50Total100.0 100.0
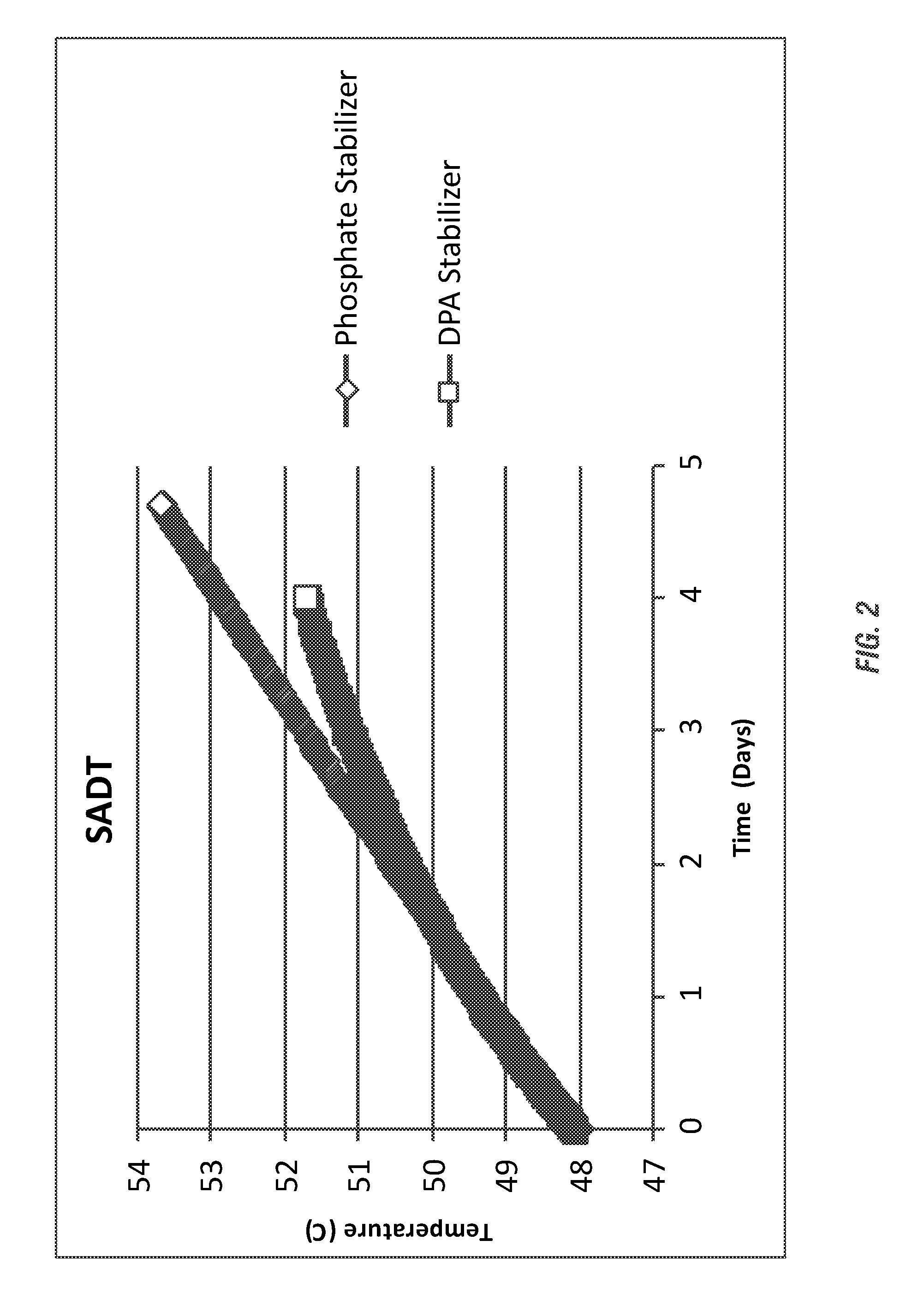
[0193]The resulting SADT from the evaluated formulations is shown in FIG. 2. The DPA-stabilized peracid composition, even under increased acidity of the peracid composition, showed a much more gradual increase in temperature (below the SADT of the peracid) in comparison to the phosphate / HEDP-stabilized peracid composition. The DPA-stabilized peracid composition would likely meet DOT standard...
example 3
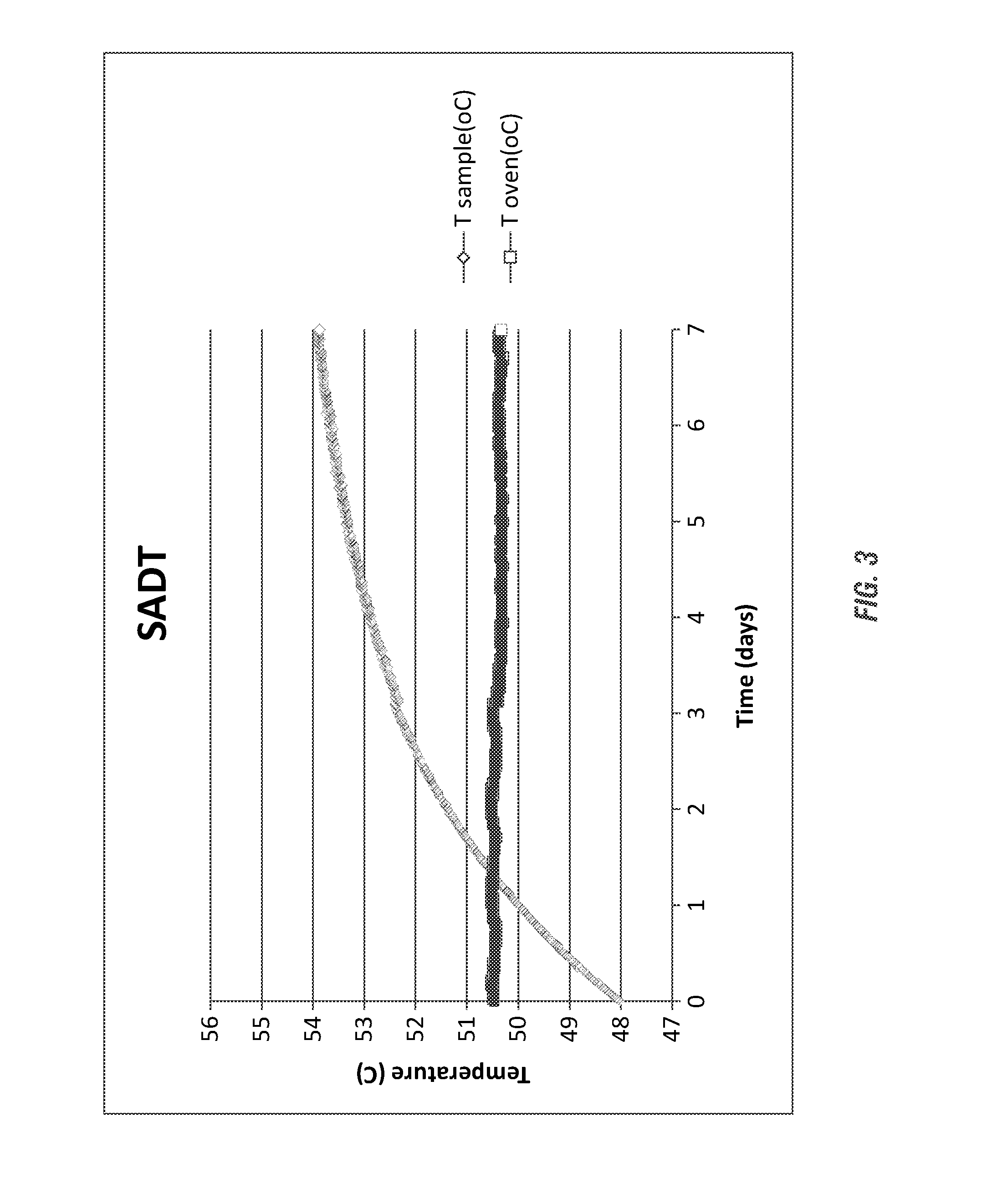
[0194]As set forth in Table 4 (and shown in FIG. 3) a strong acid containing a reduced hydrogen peroxide concentration was evaluated by the H4-SADT protocol in a Dewar flask modeling a 300 gallon IBC or plastic tote.
TABLE 4CompositionWt %DPA 0.05HEDP (60%) 0.0Sulfonated oleic acid (70%) 1-10Octanoic acid 1-10Acetic acid 2-20Sodium xylene sulfonate (40%)1-5Sodium cumene sulfonate (96%)1-5H2O2 (35%)35Al2(SO4) 3•18H2O0.05-2.0 H2SO4 (96%)18Deionized Water20-50Total 100.0
[0195]The evaluation of the stability of the composition was conducted and despite the presence of the ˜17% sulfuric acid, the composition containing the DPA stabilizing agent was stabilized. The stabilization of the composition, as shown in FIG. 3, illustrates the stabilization of the solution temperature only exceeding the 50° C. ambient temperature by 4.7° C. by day 7. Beneficially, these results qualify the highly acidic peracid composition for shipping and storage in a 300 gallon tote without refrigeration, demon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Self Accelerating Decomposition Temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com