Methods and devices for treatment of vascular defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
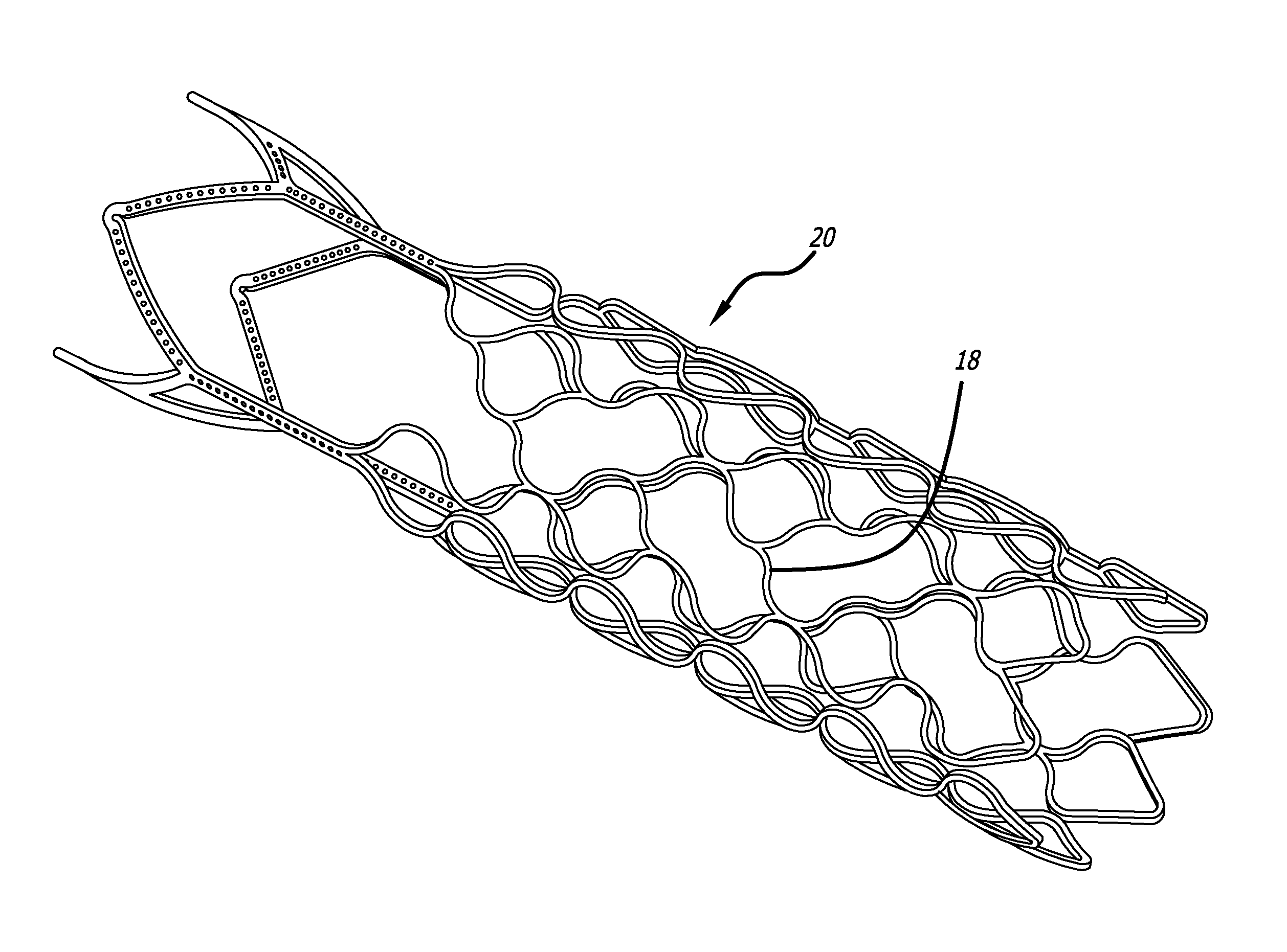
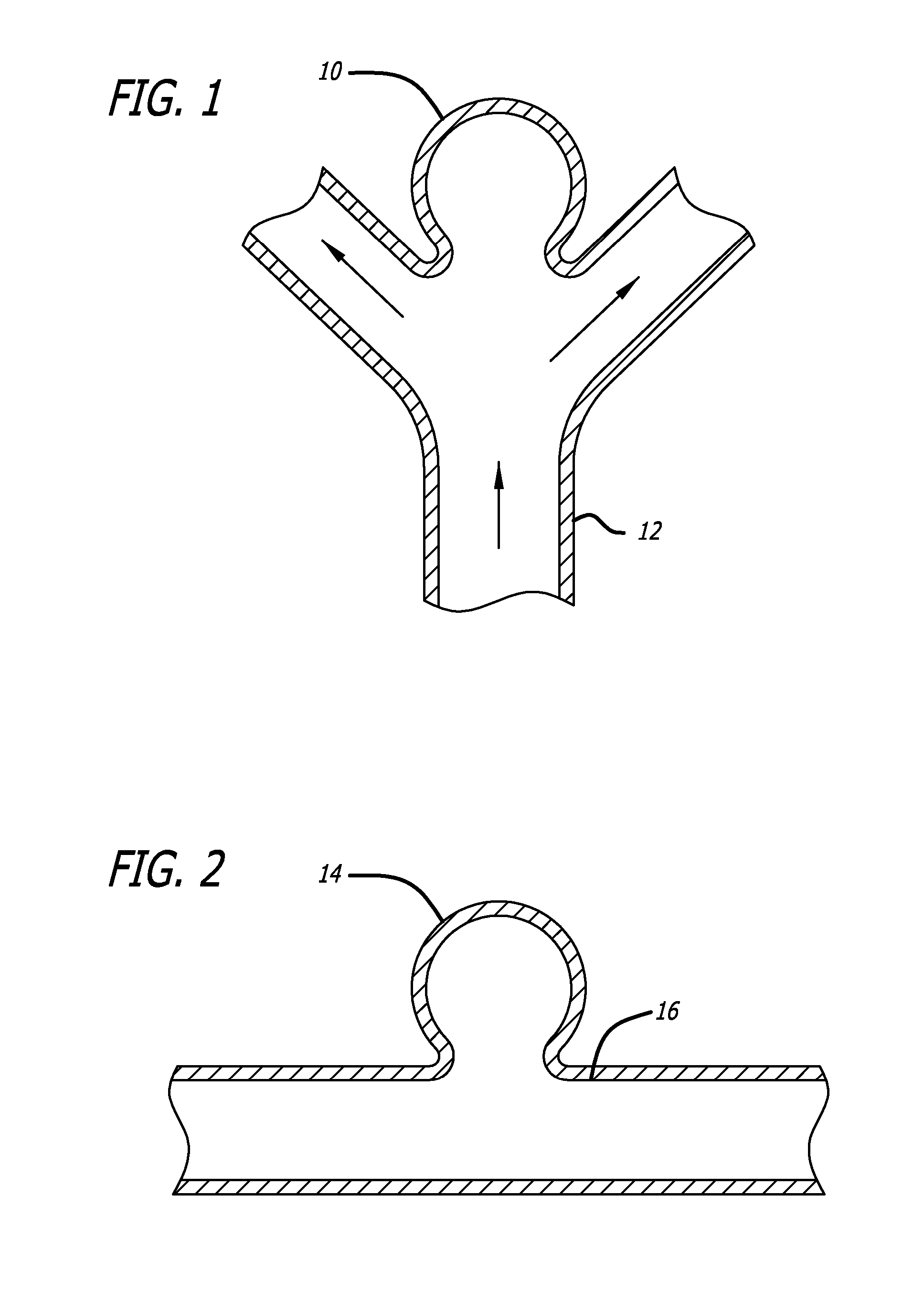
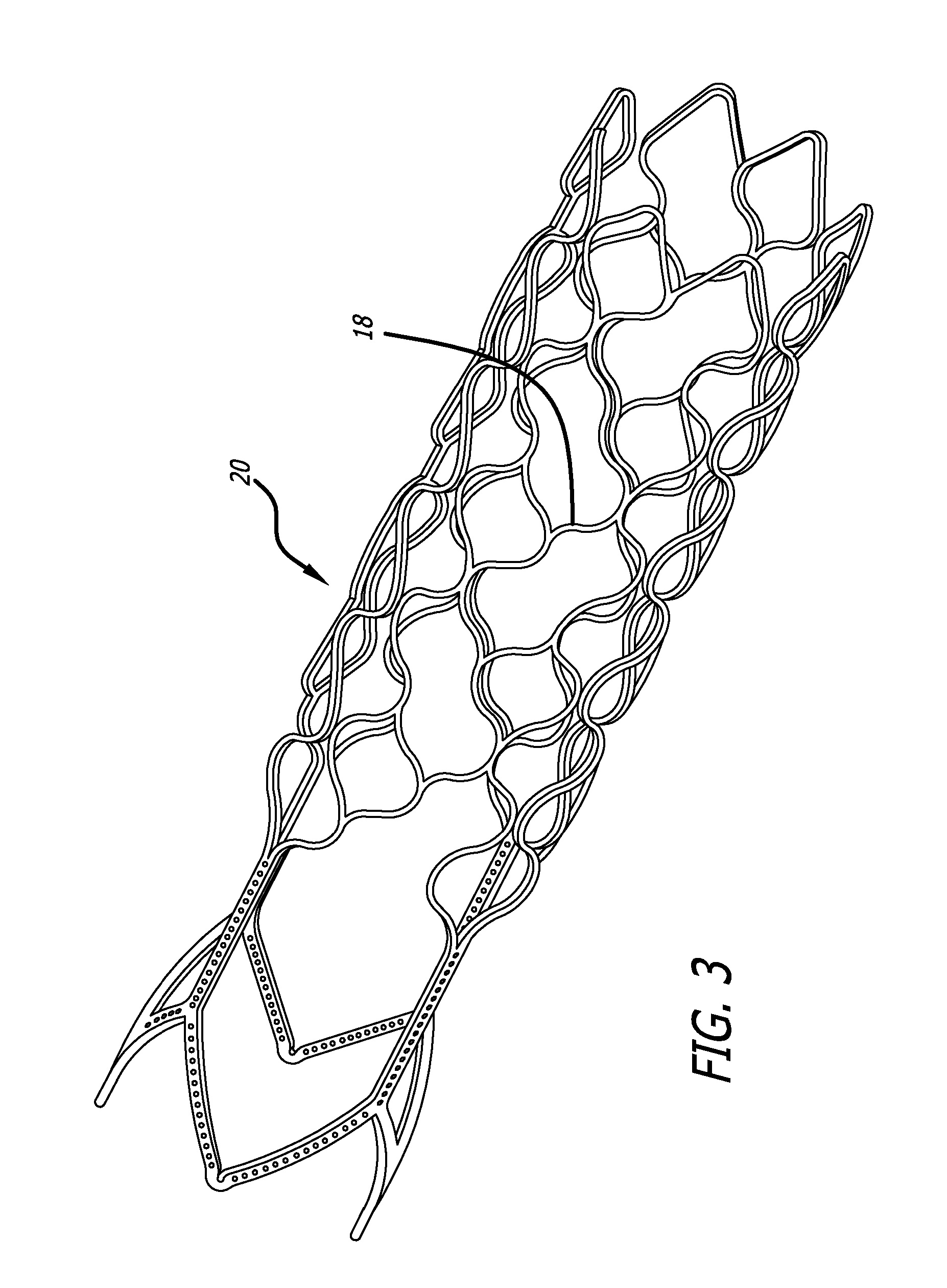
embodiment 20
The defect spanning portion may be formed of a sheet material or fabric that is attached to the one or more surfaces of the support structure. Alternatively, the defect spanning portion may be fabricated as an integral part of the overall structure. These integral members may be cut by laser, photochemical etching or electrical discharge machining (EDM). In some embodiments, the defect spanning strut members 18 have at least one portion that has a wave-like shape as shown in the support structure embodiment 20 in FIG. 3. For some embodiments, material may be attached to the structure such that it substantially reduces the size of the fenestrations or cells and thus reduces the porosity in that area. For example, coatings, filaments, fibers, wires, struts, ribbons, sheet or fabric may be connected to portions of the structure to create small fenestrations or cells and thus higher density of the defect spanning portion (as shown in FIG. 18 below). Active materials such as a responsive...
embodiment 400
FIGS. 33 and 34 illustrate an embodiment of a device 420 that may have the same or similar features, dimension and materials as those of the device of FIGS. 29-32. The device for treatment of a patient's vasculature 420 shown in FIG. 33 includes a substantially flattened first end 422 or side of the device a support structure 424 and a defect spanning structure 426. FIG. 34 illustrates an outline of an embodiment of a device for treatment of a patient's vasculature 440 that includes a trunk portion 442 extending from a first end 444 of the device. The trunk portion 442 is a somewhat cylindrical extension extending from the nominal globular or spherical shape of the device. For some of these embodiments, the detachment hub 412 may be recessed so that the profile of the device in the blood vessel lumen is reduced. The device embodiments 420 and 440 of FIGS. 33 and 34 may be delivered and deployed in the same manner and with any of the same devices and methods as those discussed above ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com