18f-saccharide-folates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
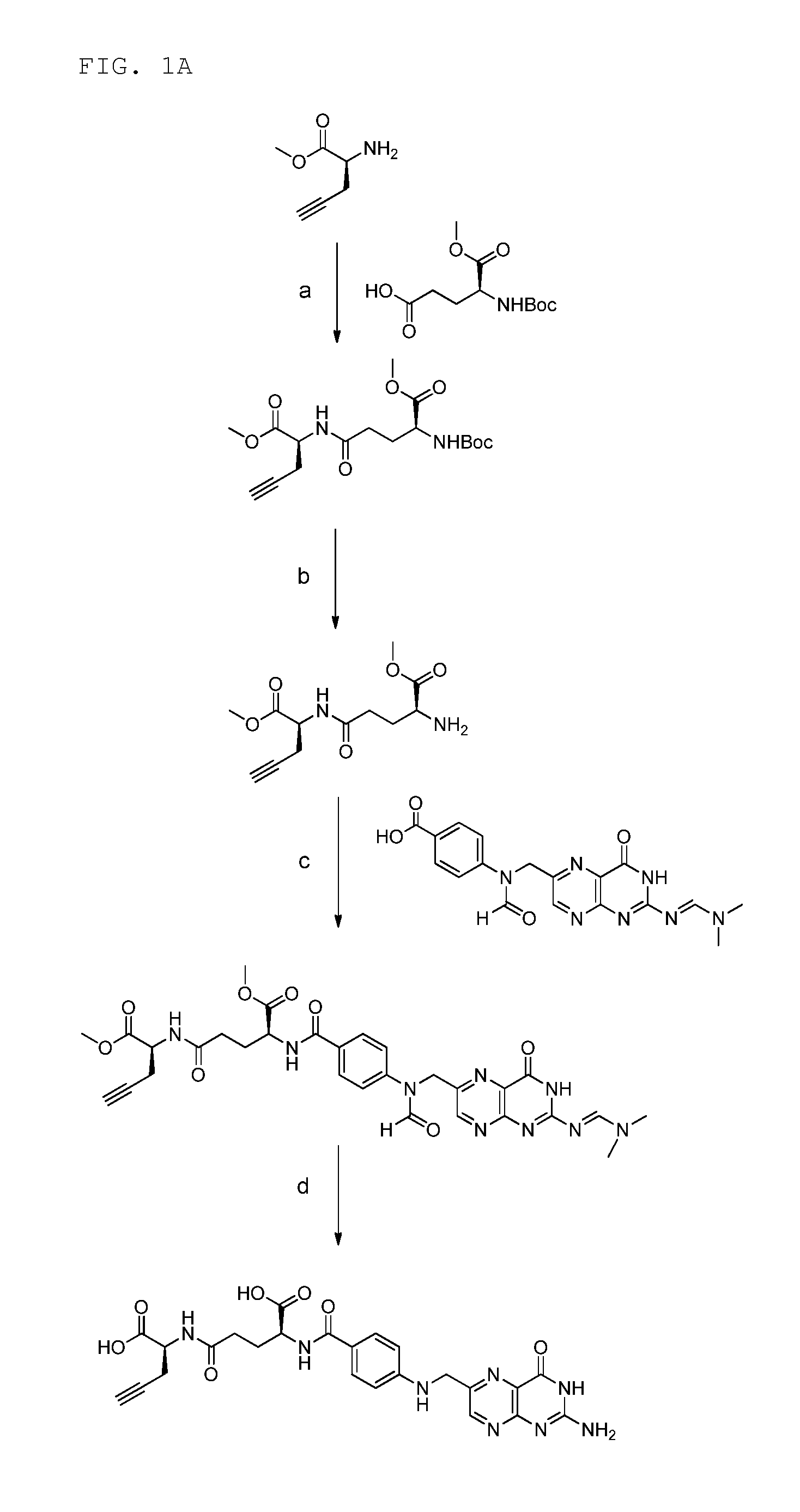
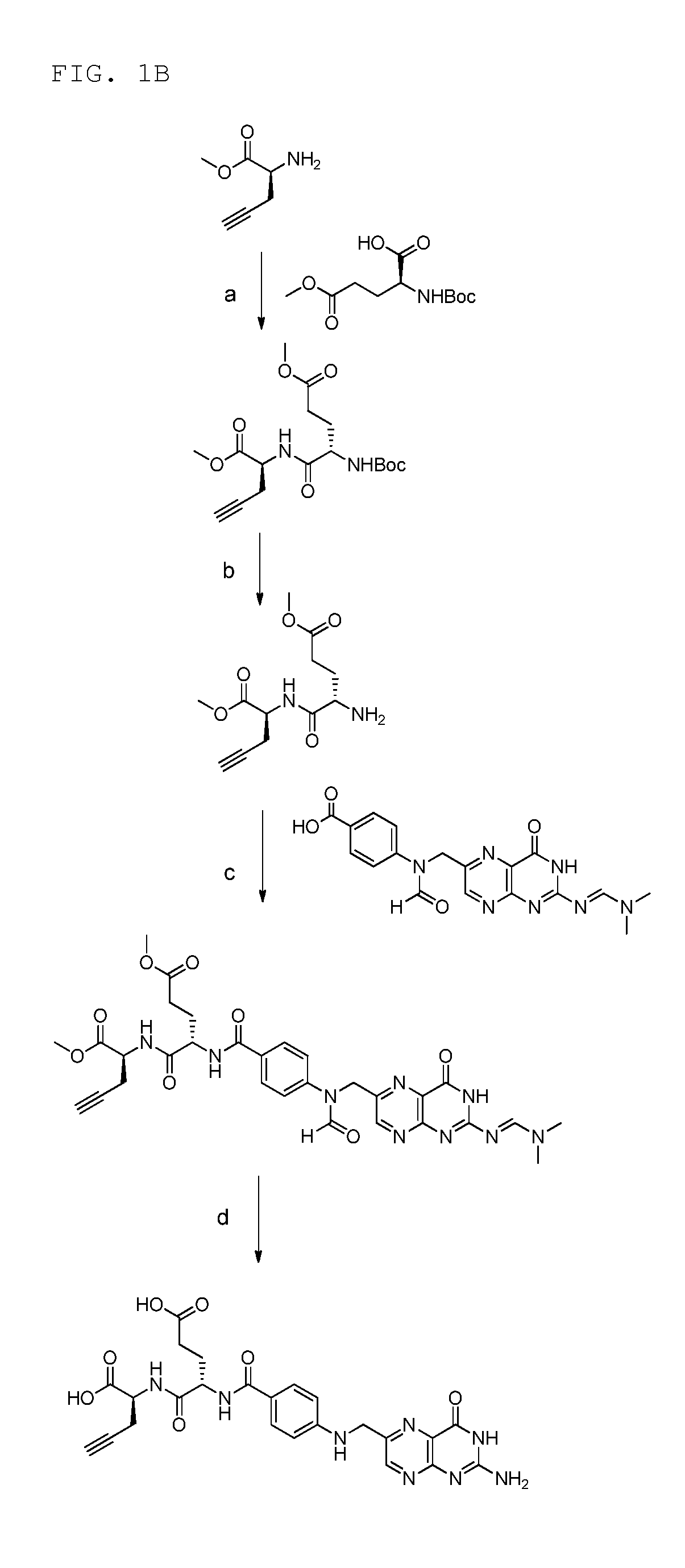
Synthesis of γ-Folate Alkyne Precursor (According to FIG. 1A)
(a) Synthesis of (S)-methyl 2-((S)-4-((tert-butoxycarbonyl)-amino)-5-methoxy-5-oxopentananamido)pent-4-ynoate (step a)
[0271]Commercial available BocGluOMe (402 mg, 1.54 mmol) was dissolved in dry DMF (4 mL) and Et3N (428 μL, 2 eq.) was added. HBTU (700 mg, 1.85 mmol) was added at 0° C. and the mixture was stirred for half an hour. The solution of the activated acid was transferred to a solution of H-Pra-OMe.HCl (205 mg, 1.62 mmol) in dry DMF (4 mL) containing Et3N (856 μL, 4 eq.) at 0° C. The mixture was stirred for 1 h at 0° C., warmed to rt and stirred over night. The product was extracted with citric acid (1 M) and ethyl acetate. The organic phase was rinsed with brine, dried over Na2SO4 and concentrated under reduced pressure. Purification was achieved by flash chromatography on silicagel with CH2Cl2 / MeOH (50:1) provided the product as a white solid (467 mg, 82%). 1H-NMR (DMSO-d6) δ / ppm 8.40 (d, 1H, J=7.3 Hz), 7.27 (d,...
example 2
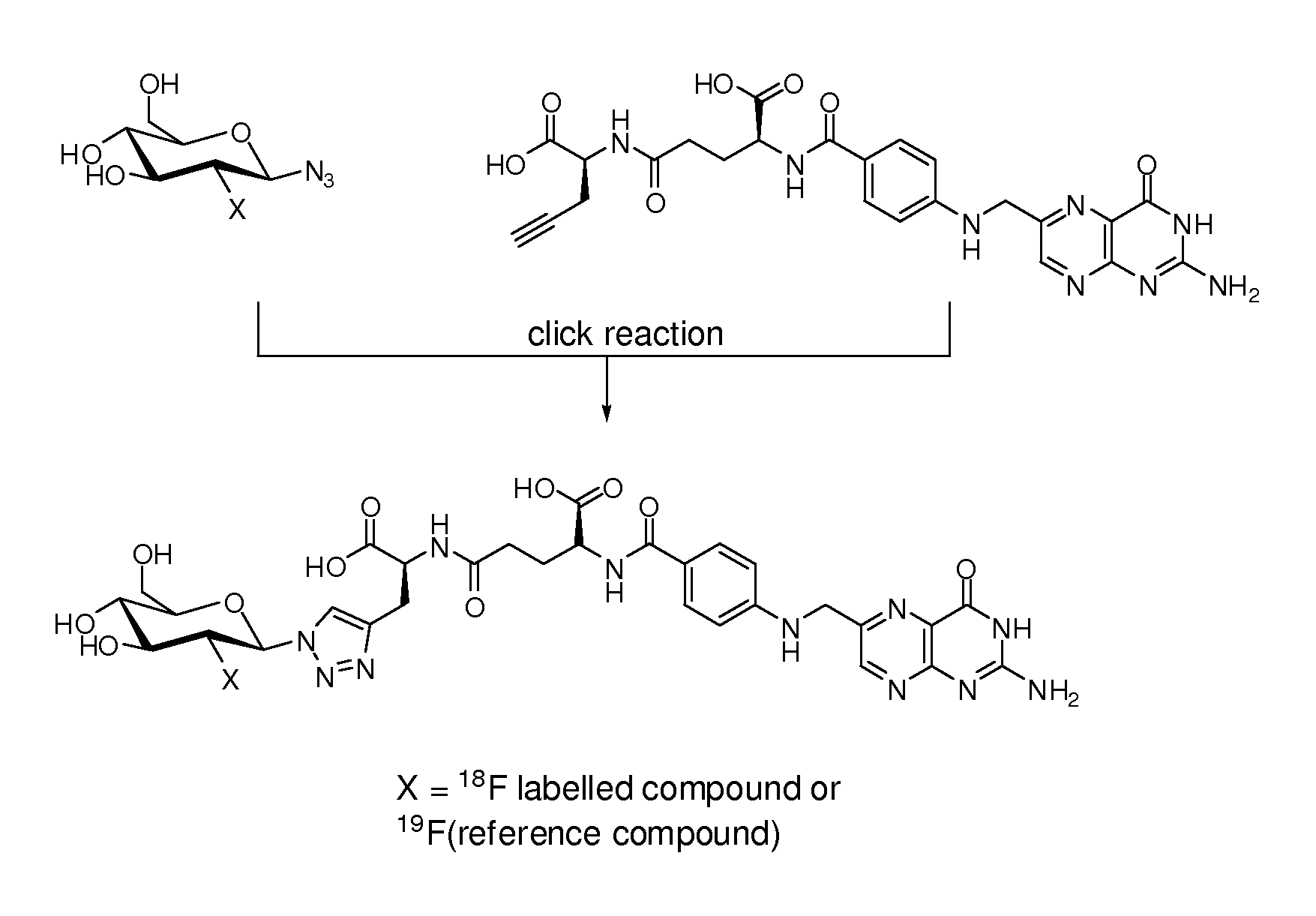
Synthesis of γ-[19F]-Glucose Folate Reference (According to FIG. 2A)
[0276]The synthesis of 2-deoxy-2-fluoroglucopyranosyl azide was prepared according to the procedure according the literature procedure (e.g. Maschauer and Prante, Carbohydr. Res. 2009).
[0277]γ-Folate alkyne (10 mg, 19 μmol) was dissolved in tert-BuOH / H2O (1:1, 1 mL) in an Eppendorf tube and 2-deoxy-2-fluoroglucopyranosyl azide (11.6 mg, 56 μmol), 0.1 M Cu(OAc)2 solution (0.1 eq., 19 μL) and 0.1 M sodium ascorbate solution (0.2 eq., 38 μL) were added. The solution was shaken at rt and 500 rpm for 1 h until complete conversion (analysis via HPLC). For isolation of the product, the mixture was submitted to semi-preparative HPLC. The desired fraction was collected and lyophilized to provide the product as a yellow powder (7.2 mg, 52%, purity according to HPLC>98%). 1H-NMR (D2O / NaOD) δ / ppm 8.74 (s, 1H), 7.98 (s, 1H), 7.61 (d, 2H, J=8.8 Hz), 6.76 (d, 2H, J=8.8 Hz), 5.89 (dd, 1H, J1=2.6 Hz, J2=9.0 Hz), 4.91 (t, 1H, J=9.0 H...
example 3
Synthesis of γ-[18F]-glucose folate (according to FIG. 2A)
[0278]The 3,4,6-tri-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannopyranosyl azide precursor used for coupling the 18F-substituted glucose via click reaction to the folate, was obtained according to literature procedures (e.g. Maschauer and Prante, Carbohydr. Res. 2009, 753; Takatani et al Carbohydr. Res. 2003, 1073).
(b) Radiosynthesis of 2-[18F]fluoroglucopyranosyl azide
[0279]To the dry 18F-fluoride-cryptate complex the precursor, 3,4,6-tri-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannopyranosyl azide (3.0 mg, 6.5 μmol), in 0.30 mL of anhydrous acetonitrile was added. The mixture was stirred for 5 min at 80° C. to afford a 18F-incorporation of maximum 75% according to radio-UPLC analysis. After 5 min of cooling and addition of 8 mL of water, the mixture was passed through a reversed-phase cartridge (Sep-Pak C18 Plus; Waters; preconditioned with MeOH and H2O). The cartridge was washed with 5 mL of water. The 18F-labelled pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Capacitance | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com