Specific delivery of agrochemicals
a technology for agrochemicals and plants, applied in the direction of immunoglobulins against plants, biocide, peptides, etc., can solve the problems of reduced efficacy of chemicals, loss of biodiversity, groundwater contamination, etc., and achieve the effect of reducing the dose of agrochemicals, maintaining the overall efficacy, and reducing the frequency of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation and Selection of VHH
[0242]Immunization of Llamas with Gum Arabic, Potato Leaf Homogenate, or Wheat Leaf Homogenate
[0243]A solution of gum arabic was prepared by weighing 5 g of gum arabic from acacia tree (Sigma) and dissolving in 50 ml water. Bradford protein assay was used to determine the total protein concentration. Aliquots were made, stored at −80° C., and used for immunization. Homogenized leaves from potato plants (Solanum tuberosum variety Désirée) or wheat plants (Triticum aestivum variety Boldus) were prepared by freezing leaves in liquid nitrogen and homogenizing the leaves with mortar and pestle until a fine powder was obtained. Bradford protein assay was used to determine the total protein concentration. Aliquots were made, stored at −80° C., and suspensions were used for immunization.
[0244]Llamas were immunized at weekly intervals with six intramuscular injections of gum arabic, homogenized potato leaves, or homogenized wheat leaves, according to standard p...
example 2
Characterization of the VHH
[0253]Single-Point Binding ELISA—
[0254]A single-point binding ELISA was used to identify clones that bind to gum arabic or plant extracts. VHH-containing extracts for ELISA were prepared as follows. 96-well plates with 100 μl per well 2×TY, 2% glucose 100 μg / ml ampicillin were inoculated from the master plates and grown at 37° C. overnight. 25 μl per well of overnight culture was used to inoculate fresh 96-well deep-well plates containing 1 ml per well 2×TY; 0.1% glucose; 100 μg / ml ampicillin. After growing at 37° C. in a shaking incubator for 3 hours, IPTG was added to 1 mM final concentration and recombinant VHH was produced during an additional incubation for 4 hours. Cells were spun down by centrifugation at 3,000 g for 20 minutes and stored at −20° C. overnight. Cell pellets were thawed, briefly vortexed, and 125 μl per well of room temperature PBS was added. Cells were resuspended on an ELISA shaker platform at room temperature for 15 minutes. Plates...
example 3
Binding of Binding Domains to Plant Surface
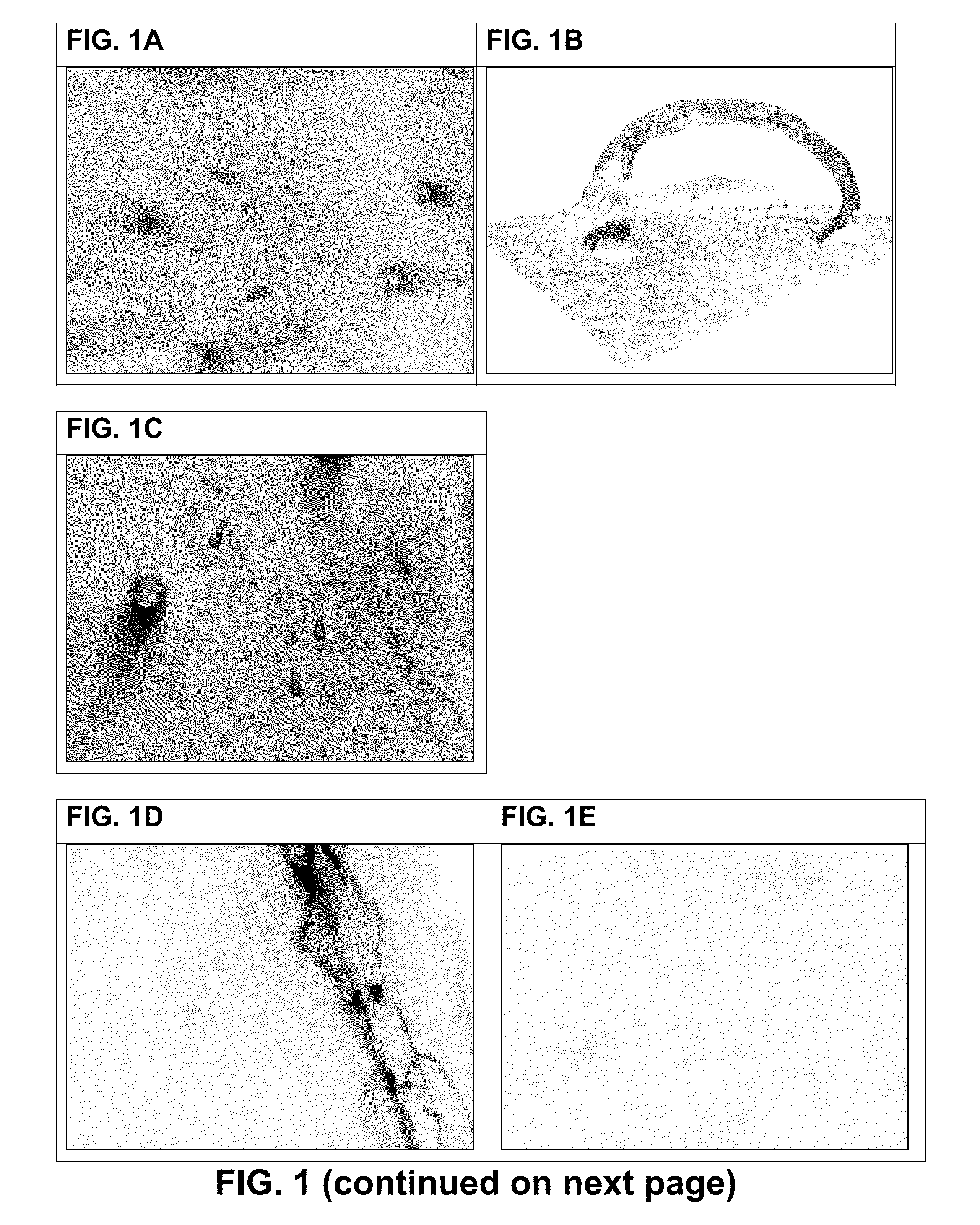
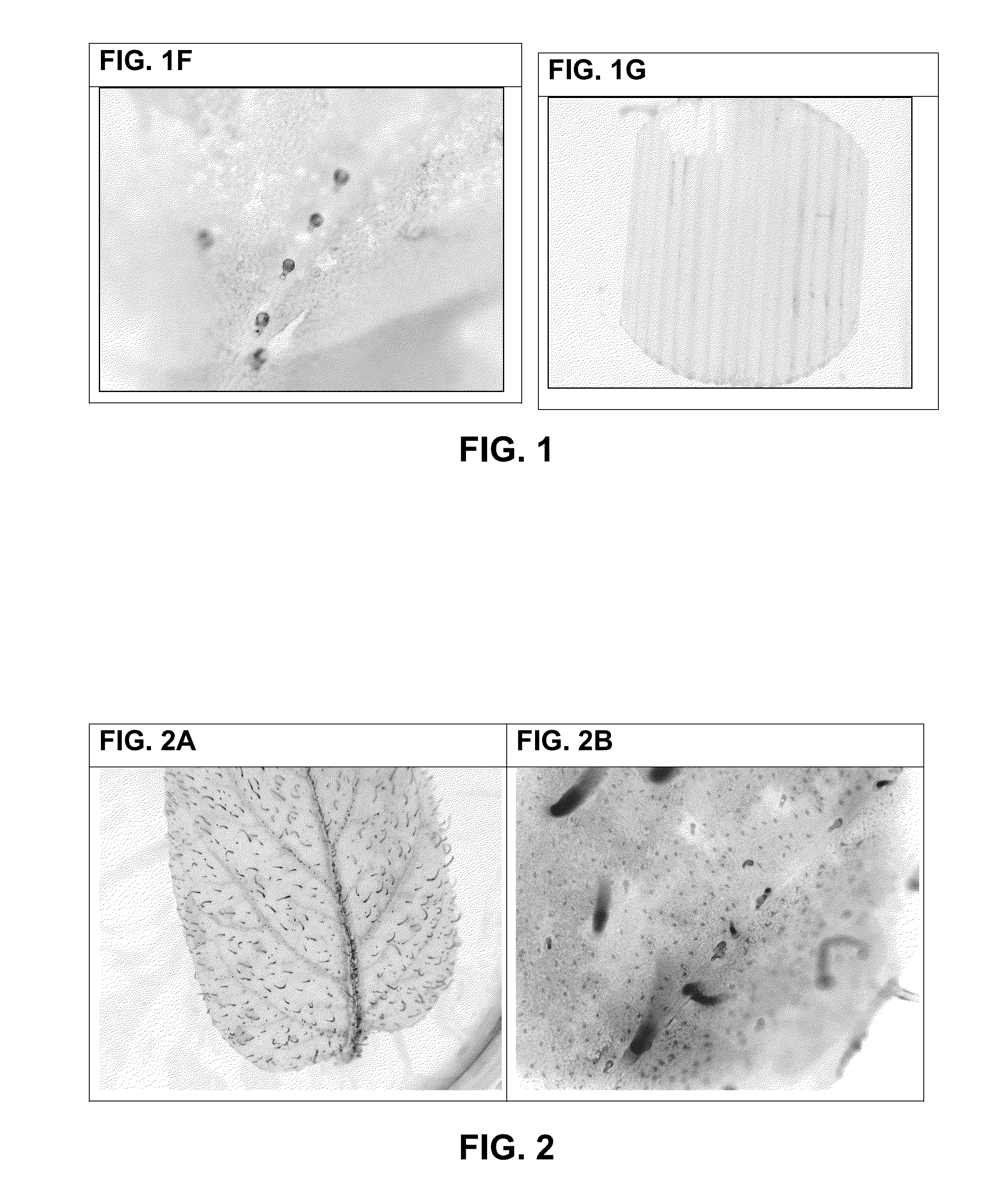
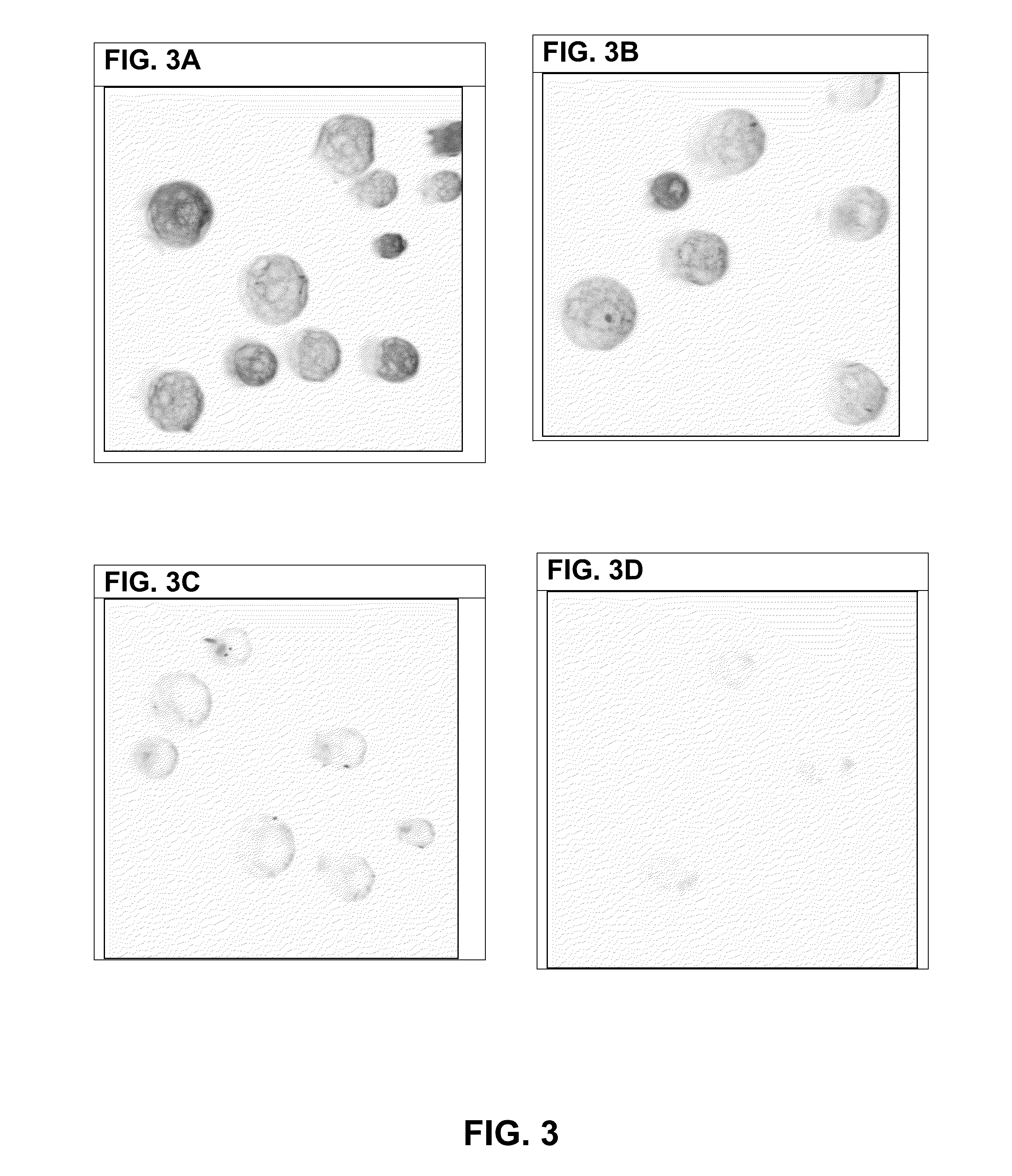
[0266]VHH Binding to Leaf Discs—
[0267]VHH binding to non-fixed leaf discs of potato (variety Désirée), black nightshade, grass, wheat or azalea was investigated. For comparison, binding of CBM3a to non-fixed leaf discs of potato (variety Désirée) was analyzed in parallel. Leaf discs were prepared by punching a fresh potato leaf with a 5 mm belt hole puncher tool. Leaf discs were put immediately in wells of a 96-well plate containing 200 μl per well 5% MPBS or PBS, and incubated for 30 minutes. Leaf discs were transferred to solutions containing 5 μg / ml VHH antibody fragment, respectively 5 μg / ml CBM3a in 2% MPBS or PBS and incubated for 60-90 minutes. Unbound VHH or CBM3a proteins were removed by washing three times with 2% MPBS or PBS. Bound VHH or CBM3a proteins were detected with incubation with monoclonal mouse anti-histidine antibodies directly conjugated with Alexa-488 fluorescent dye (Abd Serotec) in 1% MPBS for 1 hour. Unbound antib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com