4-methylpyrazole formulations for inhibiting ethanol intolerance
a technology of ethanol intolerance and formulation, applied in the direction of anti-noxious agents, drug compositions, biocide, etc., can solve the problems of increased long-term health risks of people who express aldh2*2 alleles, side effects, nausea, headache and nausea, etc., to prevent, reduce or ameliorate symptoms, the effect of reducing the likelihood or risk of a subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0062]Provided herein are methods, compositions and formulations useful for reducing or ameliorating the severity of or preventing, an adverse physiological symptom associated with acetaldehyde accumulation accompanying ethanol consumption in a subject with reduced or absent aldehyde dehydrogenase subtype 2 activity. The subject may express specific polymorphisms of the aldehyde dehydrogenase subtype 2 (ALDH2) gene and the alcohol dehydrogenase subtype 2 (ADH2) gene. For example, the subject may be a carrier of the variant ALDH2*2 allele of the ALDH2 gene, and may further be a carrier of the variant ADH2*2 allele of the ADH2 gene, as described herein.
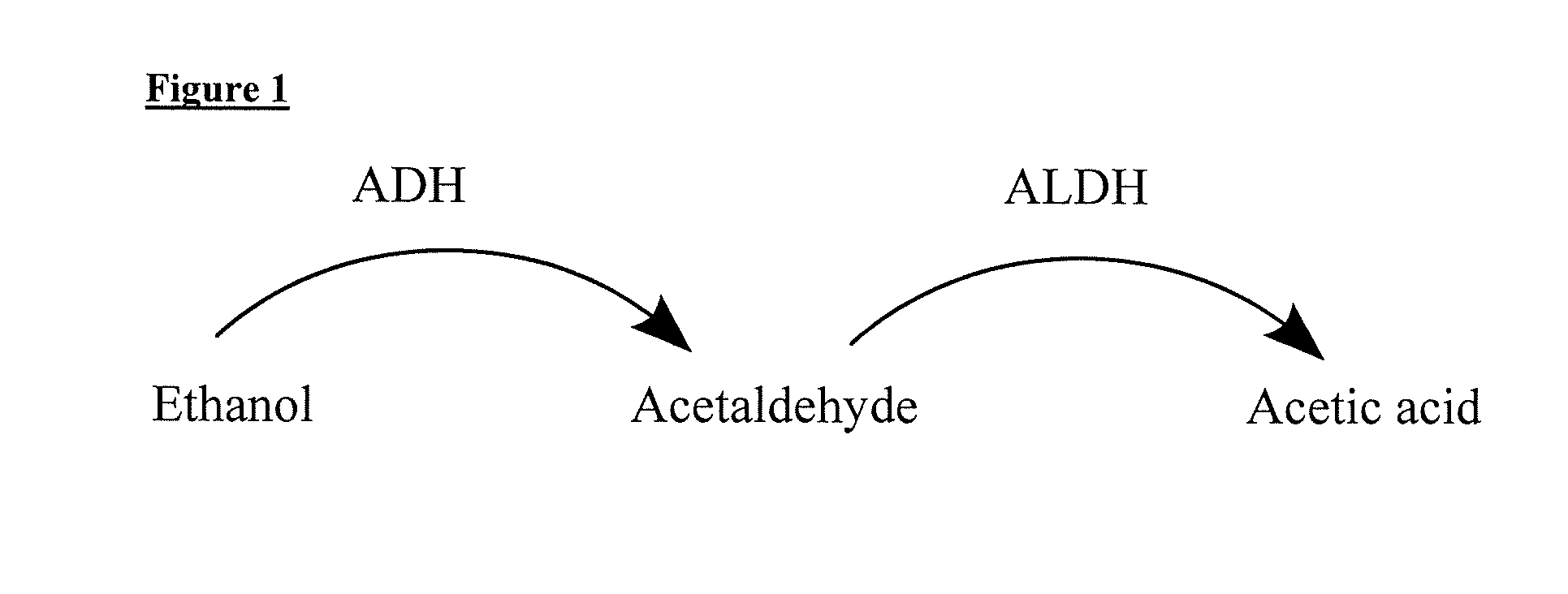
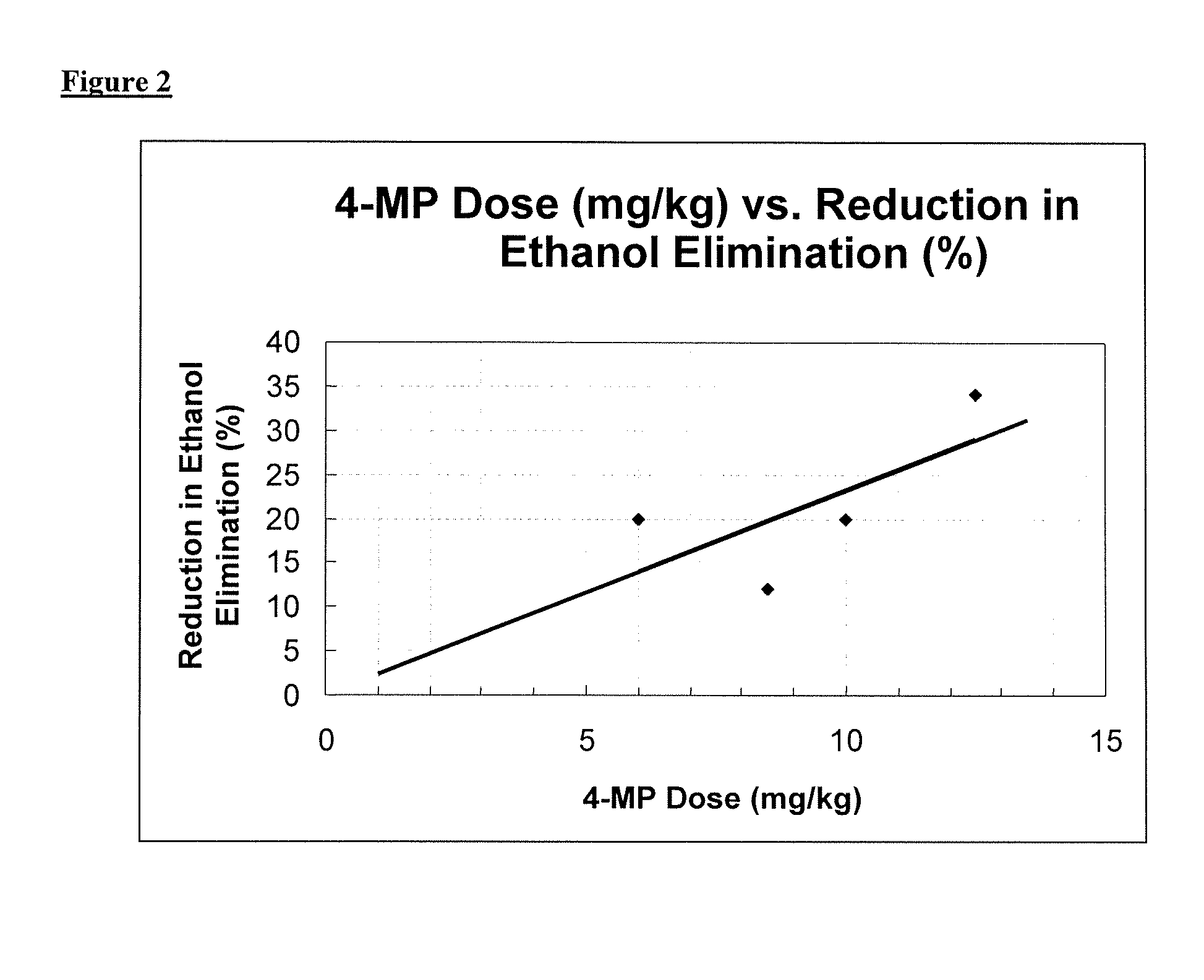
[0063]As explained below, the methods provided comprise the administration of 4-MP or a physiologically acceptable salt of 4-MP. Without intending to be bound by any particular theory or mechanism, it is believed that 4-MP acts to inhibit alcohol dehydrogenase (ADH) to reduce the accumulation of acetaldehyde production that results from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| body mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com