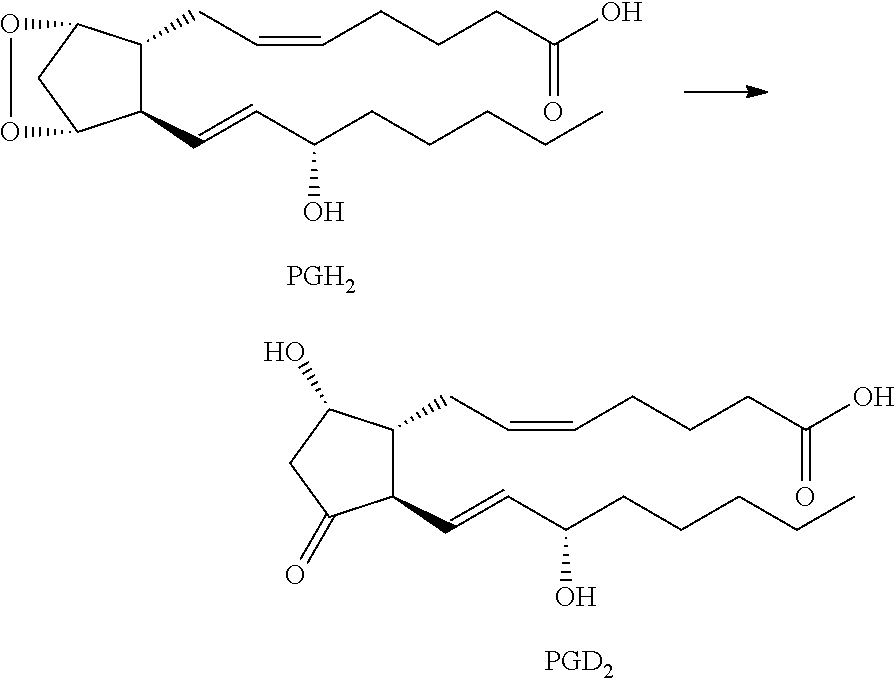
Multiheteroaryl compounds as inhibitors of h-pgds and their use for treating prostaglandin d2 mediated diseases
a technology of prostaglandin d2 and multiheteroaryl compounds, which is applied in the direction of immunological disorders, drug compositions, biocides, etc., can solve the problems of many existing medicines suffering from side effects, headache, sleepiness, sedation, etc., and failing to address a broader range of symptoms that affect patient quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-phenyl-5-(2-phenyl-1H-imidazol-5-yl)pyrimidine
[0255]
Step A: Preparation of 5-bromo-2-phenylpyrimidine
[0256]
[0257]A mixture consisting of 5-bromo-2-iodopyrimidine (Bridge Organics, 10.0 g, 35.1 mmol), benzene boronic acid (Alfa Aesar, 4.25 g, 35.1 mmol), tetrakis(triphenylphosphine)palladium (Strem, 0.405 g, 0.351 mmol), toluene (150 mL), and a 2 M aqueous sodium carbonate solution (35 mL) was stirred at 115° C. (degrees Celsius) under a nitrogen atmosphere for 16 hours. After cooling to room temperature, the mixture was partitioned between chloroform (250 mL) and brine (200 mL). The phases were separated and the organic phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give an orange oil (9.1 g). The crude product was purified by flash silica column chromatography. Elution through a 500-g Analogix® flash silica cartridge with 100% hexanes afforded the title intermediate as a white solid (3.15 g, 38% yield). Rf 0.69 with 9:1...
example 2
Preparation of 2-phenyl-5-(2-phenyl-1H-imidazol-5-yl)pyridine
[0265]
[0266]The title compound was prepared by the method described in Example 1, except that commercially available 2-iodo-5-bromopyridine was used instead of 2-iodo-5-bromopyrimidine in Step A; Rf 0.53 with 95:5 v / v dichloromethane-methanol; melting point 206° C.; 1H-NMR (400 MHz; DMSO-d6) δ 9.15 (s, 2H), 8.26 (dd, 2H), 8.14-7.94 (m, 5H), 7.52-7.34 (m, 5H); MS (ESI+) m / z 298.1 (M+1); H-PGDS FPBA IC50: 0.48 μM; H-PGDS inhibitor EIA IC50: 0.097 μM.
example 3
Preparation of 5-(1-methyl-2-phenyl-1H-imidazol-5-yl)-2-phenylpyrimidine
[0267]
[0268]The title compound was prepared by the method described in Example 1, Steps A-C, except that iodomethane was used instead of benzyl bromide in Step B; Rf 0.36 with 1:1 v / v ethyl acetate-hexane; melting point 211° C.; 1H-NMR (400 MHz; DMSO-d6) δ 9.17 (s, 2H), 8.41 (m, 2H), 7.75 (dd, 2H), 7.6-7.27 (m, 7H); MS (ESI+) m / z 313.2 (M+1); H-PGDS FPBA IC50: 0.625 μM; H-PGDS inhibitor EIA IC50: 0.25 μM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com