Metal-air cell with ion exchange material
a metal-air battery and ion exchange technology, applied in the field of electrochemical metal-air batteries, can solve the problems of limiting the evaporation of the electrolyte solution (i.e., the ionically conductive medium) of the electrolyte solution, and posed significant detriment to the energy density of most batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
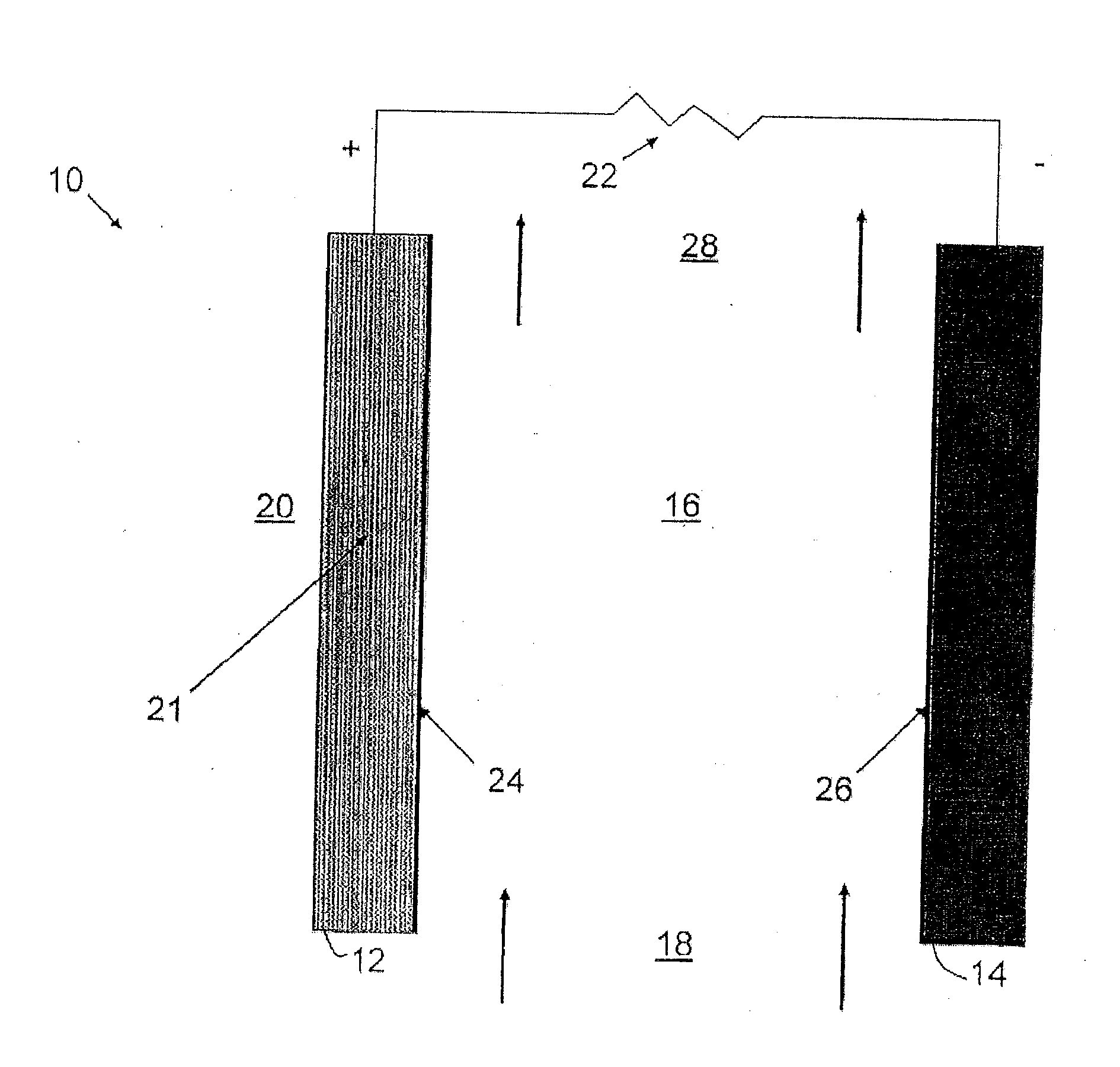
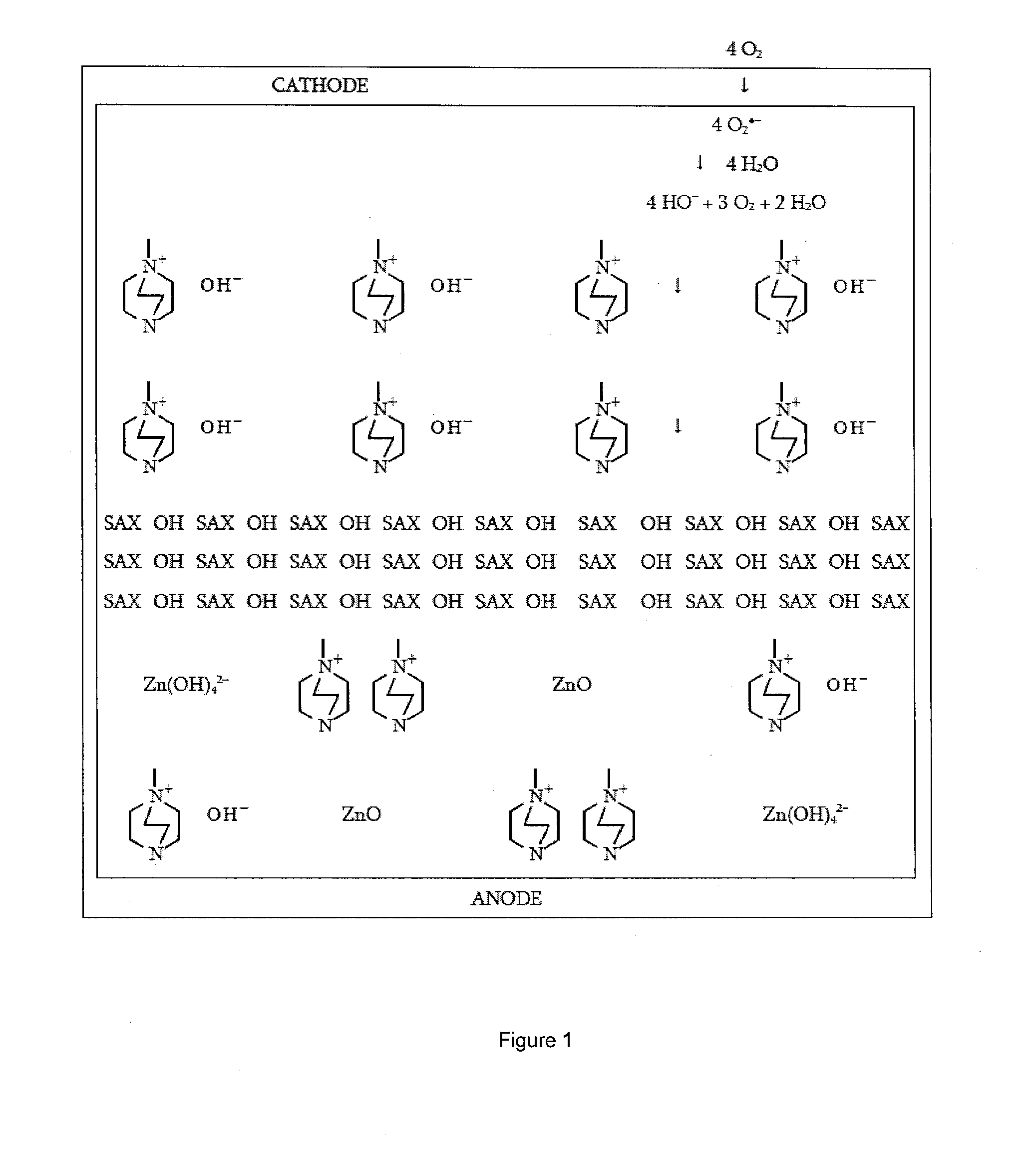
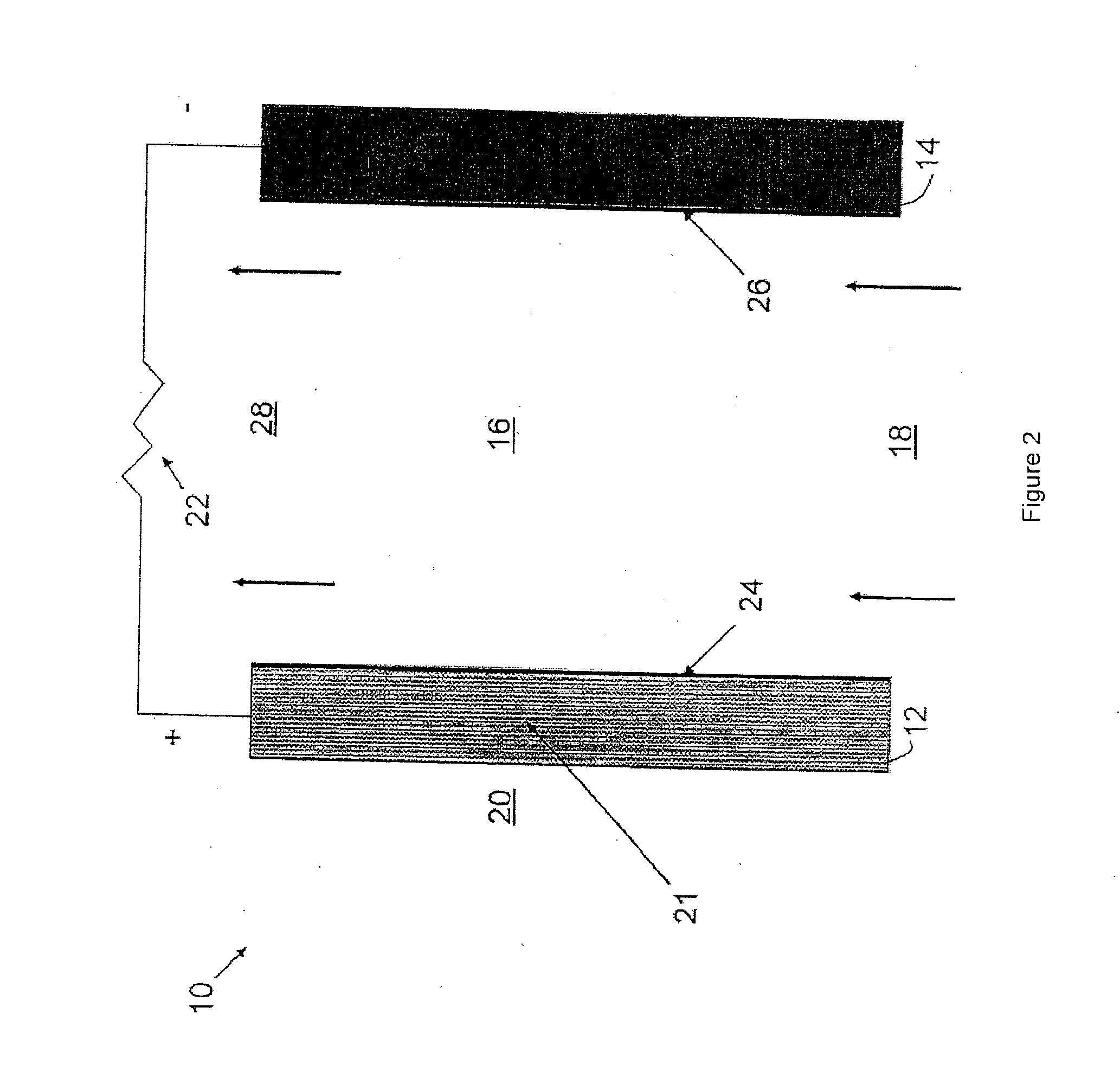
[0024]According to an embodiment, an ionic exchange material comprising a polymer derivatized with a cation is a solid electrolytic component of any electrochemical cell. According to another embodiment, an ionic exchange material comprising a polymer comprising ketone or sulfone groups derivatized with 1,4-diazabicyclo[2.2.2]octane (DABCO) is a solid electrolytic component of any electrochemical cell, including but not limited to a fuel cell, a metal-air battery, an O2 separator, a chloralkali cell, or an electrolyzer.
[0025]According to another embodiment, a metal-air battery includes an ion exchange material that is intended to help address challenges relating to undesirable material transport within the battery. According to one embodiment, the ion exchange material may be provided in the form of a separator or membrane, or may be included within a conventional separator. According to other embodiments, the ion exchange material may be provided as a film or membrane that is appli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conduction | aaaaa | aaaaa |
| ionically conductive | aaaaa | aaaaa |
| ionic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com