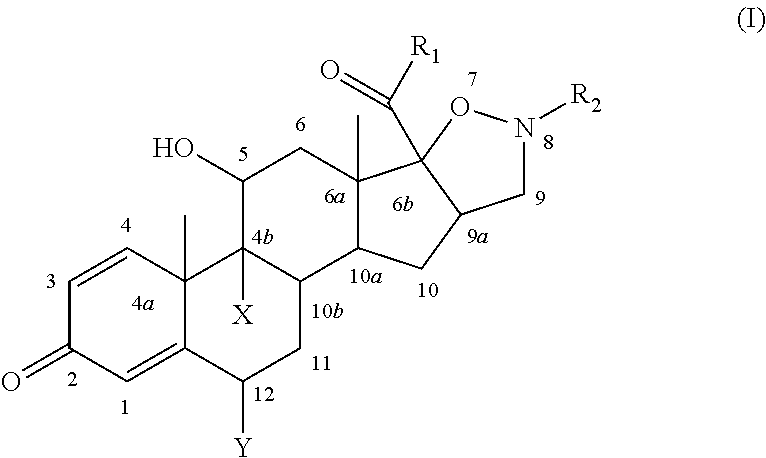
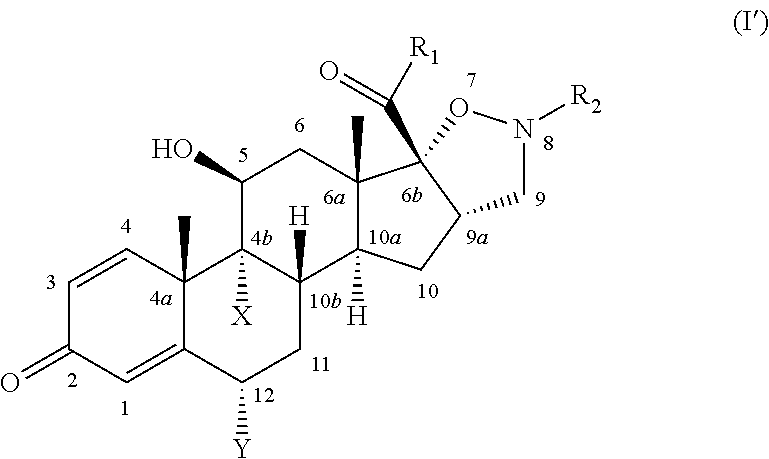
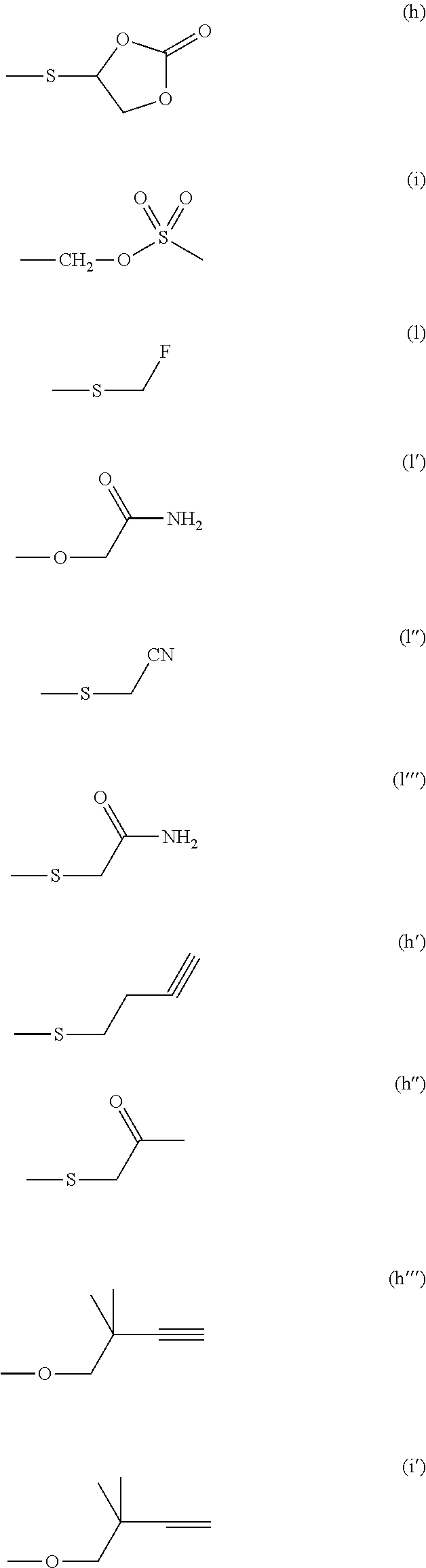Isoxazolidine derivatives
a technology of isoxazolidine and derivatives, applied in the field of new antiinflammatory and antiallergic compounds, can solve the problems of limited duration of action, and achieve the effects of improving the development ability, pharmacokinetic or pharmacodynamic characteristics, and reducing the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of acetic acid (8S,9S,10R,13S,14S,17R)-17-(2-acetoxy-acetyl)-10,13-dimethyl-3,11dioxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl ester (intermediate 2)
[0371]
[0372]To a suspension of acetic acid 2-((10R,13S,17R)-17-hydroxy-10,13-dimethyl-3,11-dioxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester (intermediate 1) (2 g, 4.99 mmol) and p-toluene sulphonic acid (PTSA) (200 mg, 1.051 mmol) in acetic acid (5 ml), at 0° C., trifluoroacetic anhydride (5 ml, 35.4 mmol) was slowly added over 10 minutes. After stirring at 0° C. for 20 minutes, the reaction mixture was stirred at RT for 3 hours.
[0373]The reaction mixture was poured in ice / water (130 ml), and the resulting mixture was extracted with DCM (2×100 ml) and AcOEt (2×100 ml). The combined organic extracts were dried over anhydrous Na2SO4 and concentrated. The crude product was purified by flash chromatography on silica gel, in gradient elutio...
example 2
Preparation of acetic acid 2-((6S,8S,9R,10S,11S,13S,14S)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15-decahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester (intermediate 5)
[0378]
[0379]To a solution of butyric acid (9R,10S,11S,13S,17R)-17-(2-acetoxy-acetyl)-9-chloro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl ester (intermediate 4) (2.48 g, 4.88 mmol) in anhydrous DMF (60 ml), under nitrogen atmosphere, potassium acetate (3.83 g, 39.0 mmol) was added and the reaction mixture was stirred at 100° C. for 1.5 hours. The cooled reaction mixture was poured into ice and brine (200 ml), and the aqueous layer was extracted with AcOEt (3×150 ml). The combined organic extracts were washed with water and brine, dried over Na2SO4, and concentrated to afford 2.55 g of crude title compound which was used in the next step without further purification.
[0380]1H NMR (300 MHz, DMSO-d6): ppm 7.29 (dd, 1H)...
example 3
Preparation of (6S,8S,9R,10S,11S,13S,14S)-17-(2-(tert-butyldimethylsilyloxy)acetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15-decahydro-3H-cyclopenta[a]phenanthren-3-one (intermediate 9)
[0386]
[0387]To a solution of 6 (300 mg, 0.793 mmol) in dry DMF (12 ml), imidazole (135 mg, 1.982 mmol) and pyridine (0.321 ml, 3.96 mmol) were added, followed by tert-butylchlorodimethylsilane (263 mg, 1.744 mmol). The reaction was stirred at room temperature for 2 hours. The conversion was complete. The reaction was diluted with AcOEt and washed with water, the aqueous layer was extracted twice with AcOEt, and then the combined extracts were dried over sodium sulphate and evaporated. The crude was purified via chromatographic column over silica gel (DCM, DCM / AcOEt 8:1) to give 365 mg (93% yield).
[0388]LC-MS (ESI POS): 493.1 (MH+)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com