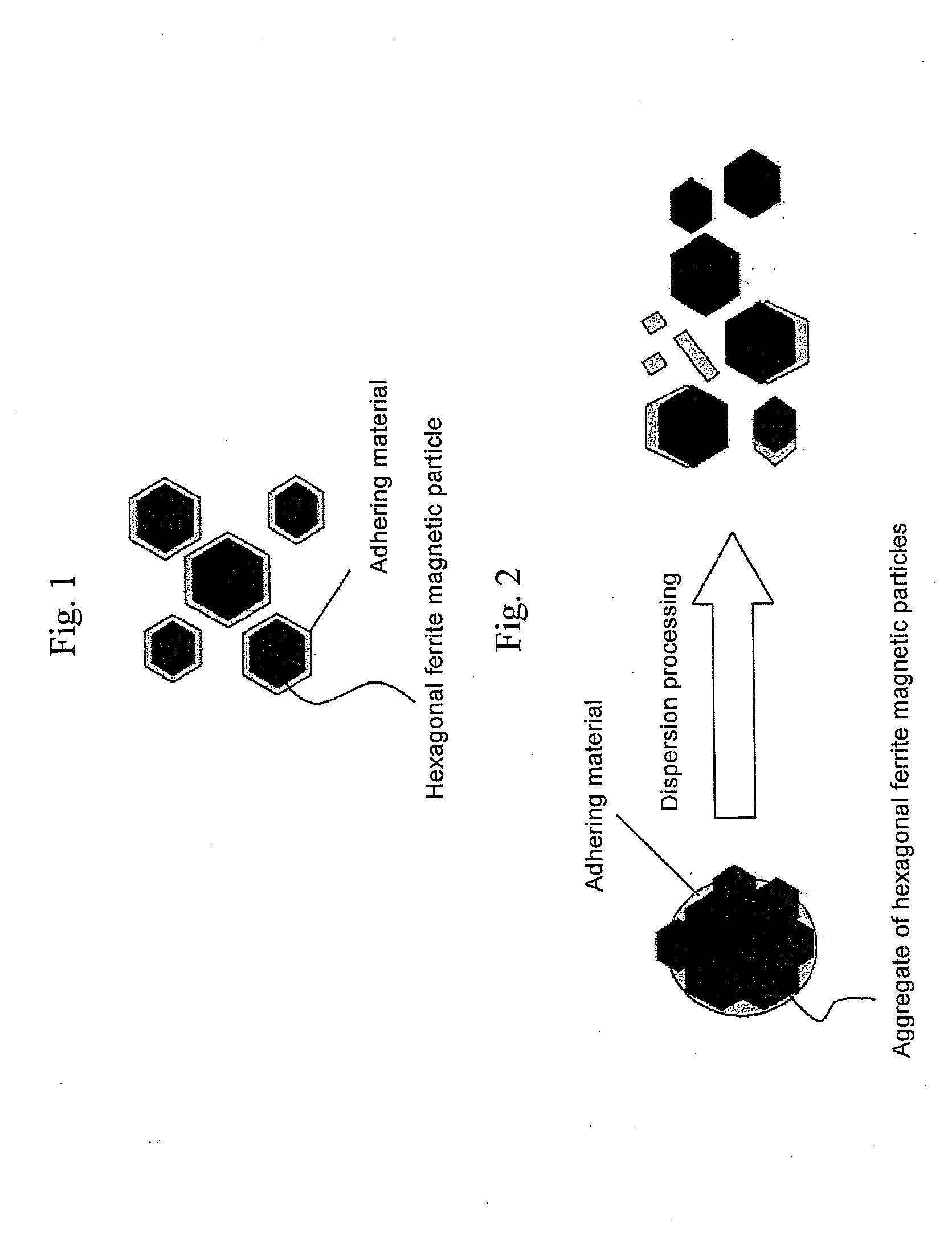
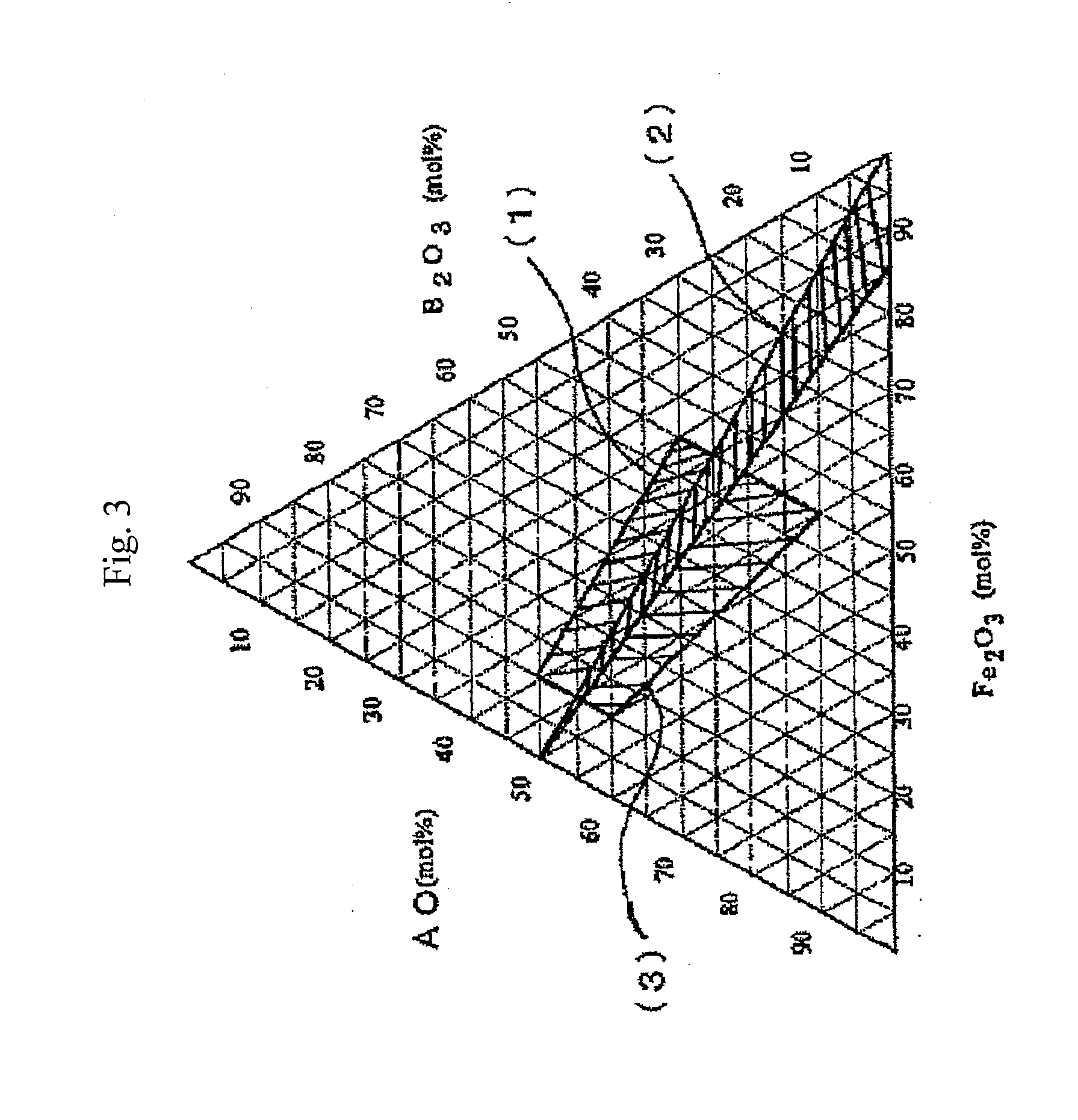
Hexagonal ferrite magnetic particle and method of manufacturing the same, and magnetic recording medium
a magnetic particle and hexagonal ferrite technology, applied in the field of manufacturing a hexagonal ferrite magnetic particle, can solve the problems of particle forming an aggregate, and hard particles of hexagonal ferrite, and achieves the effects of reducing the abrasion of the dispersion medium, high coercive force, and high density recording
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0095]Prescribed quantities of H3BO3 for B2O3, Al(OH)3 for Al2O3, BaCO3 for BaO, and Fe2O3 were weighed out so as to yield, based on conversion to the oxide, 23.0 molar percent of B2O3, 8.7 molar percent of Al2O3, 37.0 molar percent of BaO, and 31.3 molar percent of Fe2O3, charged to a mixer, and mixed. The mixture was charged to a two-liter platinum crucible, melted, and cooled with water-cooling rolls, yielding an amorphous material. A 600 g quantity of the amorphous material obtained was charged to an electric furnace, heated by 4° C. / minute to 720° C., and maintained at that temperature for five hours to cause hexagonal ferrite to crystallize (precipitate). Next, 600 g of the heat treated product after the crystallization was coarsely pulverized in a mortar and charged to a three-liter pot mill. Pulverization was conducted for four hours in a ball mill with 5 kg of Zr balls 5 mm in diameter and 1.2 kg of pure water. Subsequently, the pulverized liquid was separated from the ball...
example 2
[0096]Prescribed quantities of H3BO3 for B2O3, Al(OH)3 for Al2O3, BaCO3 for BaO, and Fe2O3 were weighed out so as to yield, based on conversion to the oxide, 26.3 molar percent of B2O3, 5.4 molar percent of Al2O3, 37.0 molar percent of BaO, and 31.3 molar percent of Fe2O3, charged to a mixer, and mixed. (A portion of the Fe was replaced with Co=0.5 at %, Zn=1.5 at %, and Nb=1 at %). The mixture was charged to a two-liter platinum crucible, melted, and cooled with water-cooling rolls, yielding an amorphous material. A 600 g quantity of the amorphous material obtained was charged to an electric furnace, heated by 4° C. / minute to 700° C., and maintained at that temperature for five hours to cause hexagonal ferrite to crystallize (precipitate). Next, 600 g of the heat treated product after the crystallization was coarsely pulverized in a mortar and charged to a three-liter pot mill. Pulverization was conducted for four hours in a ball mill with 5 kg of Zr balls 5 mm in diameter and 1.2 ...
example 3
[0097]Prescribed quantities of H3BO3 for B2O3, Al(OH)3 for Al2O3, BaCO3 for BaO, and Fe2O3 were weighed out so as to yield, based on conversion to the oxide, 30.4 molar percent of B2O3, 1.3 molar percent of Al2O3, 37.0 molar percent of BaO, and 31.3 molar percent of Fe2O3, charged to a mixer, and mixed. (A portion of the Fe was replaced with Zn=1.5 at % and Nb=0.75 at %). The mixture was charged to a two-liter platinum crucible, melted, and cooled with water-cooling rolls, yielding an amorphous material. A 600 g quantity of the amorphous material obtained was charged to an electric furnace, heated by 4° C. / minute to 660° C., and maintained at that temperature for five hours to cause hexagonal ferrite to crystallize (precipitate). Next, 600 g of the heat treated product after the crystallization was coarsely pulverized in a mortar and charged to a three-liter pot mill. Pulverization was conducted for four hours in a ball mill with 5 kg of Zr balls 5 mm in diameter and 1.2 kg of pure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com