Method for preparing lactames, comprising a photonitrosation step, followed by a beckmann transposition step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
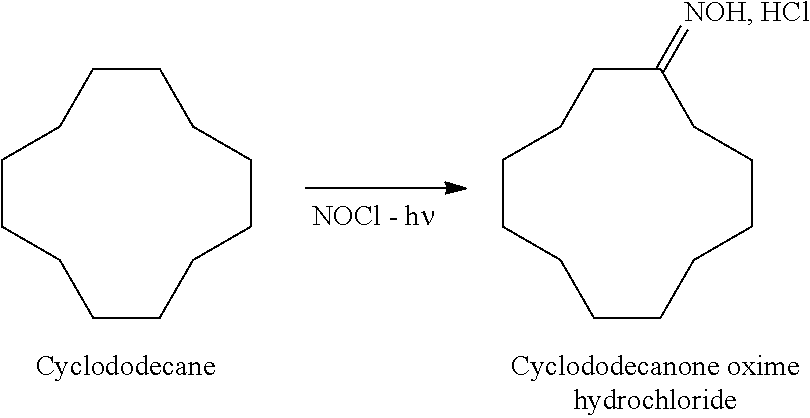
Photonitrosation of Cyclododecane by Means of LEDs
[0073]3806 g of a solution containing 32% by mass of cyclododecane in carbon tetrachloride and 200 g of sulfuric acid at 90% are introduced, with stirring, into a two-liter reactor equipped at its center with a bundle of 80 Luxeon LXML-PL01-0030 LEDs from the company Philips Lumileds, each supplying 30 lumens (for a current of 350 mA) and emitting a monochromatic light centered at 590 nm, the lamps are turned on, and then 10 l / h of anhydrous hydrochloric acid gas and 10 l / h of nitrosyl chloride are introduced continuously for 3 h, while cooling the reaction medium such that the temperature does not exceed 25° C.
[0074]The selectivity of the reaction, expressed by the ratio of the percentage of cyclododecanone oxime to the sum of the percentage of cyclododecanone oxime and of by-products of the reaction, assayed by HPLC in the sulfuric acid phase, is 89%, and therefore greater than that observed when using the mercury-vapor or sodium-v...
example 2
Beckmann Rearrangement / Dechlorination
[0075]90% sulfuric acid is injected, at a flow rate of 1 1 / h and at ambient temperature, with a Grundfos DME-2-18 feed pump into a microstructure of “DT” type having an internal volume of 9 ml (reactive circuit), manufactured by the company Corning and described, for example, in the article: Chem. Eng. Technol. 2008, 31, No. 8, 1146-1154 by P. Barthe et al.[0076]The heat-transfer fluid circuit is injected, at a flow rate of 6 with oil which is at 205° C. by means of a Lauda Integral XT 350 HW bath. Once the temperature of 200° C. has been reached at the outlet of the microstructure in the heat-transfer fluid circuit, the injection of sulfuric acid is stopped and a solution containing 30.1% (by mass) of cyclododecanone oxime (determined by HPLC) in 90% sulfuric acid is injected at a flow rate of approximately 2.5 1 / h by means of two Grundfos DME-2-18 pumps mounted in parallel.[0077]After 25 minutes during which 1017.9 g of oxime solution have been...
example 3
Beckmann Rearrangement / Dechlorination
[0081]90% sulfuric acid is injected, at a flow rate of 1 l / h and at ambient temperature, with a Grundfos DME-2-18 feed pump, into 4 microstructures, mounted in series, of “DT” type; each having an internal volume of 9 ml (reactive circuit), manufactured by the company Corning and described, for example, in the article Chem. Eng. Technol. 2008, 31, No. 8, 1146-1154 by P. Barthe et al.[0082]- The heat-transfer fluid circuit is injected, at a flow rate of 6 I / min, with oil which is at 190° C. by means of a Lauda Integral XT 350 HW bath. Once the temperature of 185° C. has been reached at the outlet of the microstructure in the heat-transfer fluid circuit, the injection of sulfuric acid is stopped and a solution containing 30.9% (by mass) of cyclododecanone oxime (determined by HPLC) in 90% sulfuric acid is injected at a flow rate of approximately 1 l / h by means of 1 Grundfos DME-2-18 pump.[0083]After 1 hour during which 766 g of oxime solution have ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com