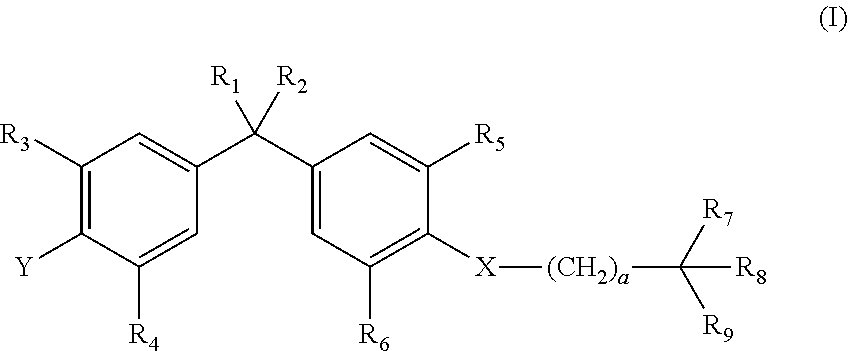
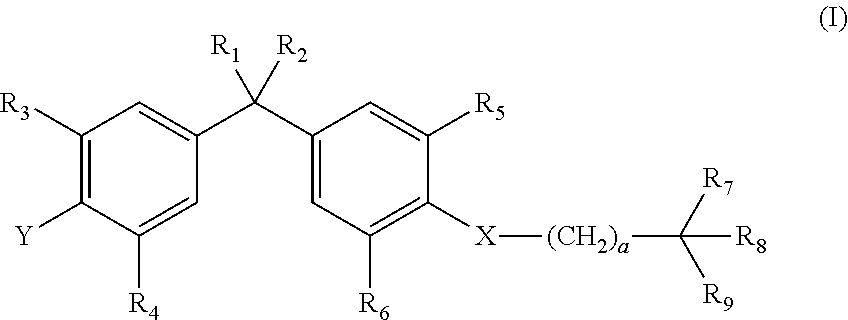
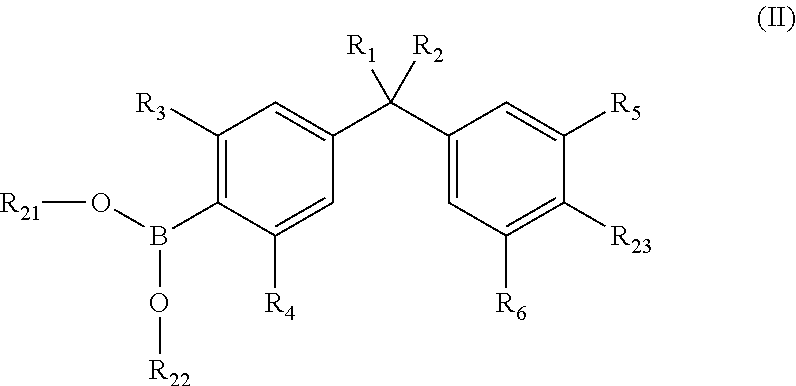
Vitamin-d-like compounds
a technology of vitamin d3 and compounds, applied in the field of new vitamin dlike compounds, can solve the problems that no such vitamin d3-like compounds have been commercially available or proceeded to clinical trials, and achieve the effect of increasing the blood calcium level and strong desirable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 4′-{1-ethyl-1-[4-((E)-3-ethyl-3-hydroxy-1-pentenyl)-3-methyl-phenyl]-propyl}-2′-methyl-biphenyl-3-carboxylic acid
[0292]
(1) Synthesis of trifluoromethanesulfonic acid 4-[1-ethyl-1-(4-hydroxy-3-methyl-phenyl)-propyl]-2-methyl-phenyl ester
[0293]Pyridine (3 mL, 37.2 mmol) and trifluoromethanesulfonic anhydride (5.7 mL, 34.7 mmol) were added to a solution of 3,3-bis[4-hydroxy-3-methylphenyl]pentane (9.0 g, 31.6 mmol) in dichloromethane (300 mL) at 0° C., and then the mixture was stirred at the same temperature for one hour. Ethyl acetate was added to the reaction mixture. The organic layer was washed with a saturated aqueous sodium bicarbonate solution and brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by a silica gel column (hexane / ethyl acetate=10 / 1 to ethyl acetate only) to give the title compound (4.9 g, 37%).
1H-NMR: 0.62 (t, 6H), 2.203 (q, 4H), 2.20 (s, 3H), 2.38 (s, 3H), 4.67 (s, 1H), 6.68 (d, 1H), 6.80-...
example 2
Synthesis of 4′-{1-ethyl-1-[4-((E)-3-ethyl-3-hydroxy-1 -pentenyl)-3-methyl-phenyl]-propyl}-2′-methyl-biphenyl-4 -carboxylic acid
[0306]
[0307]The title compound was synthesized by the same method as in Example 1-(7) using 4-carboxyphenylborane acid and the raw material 1-(6).
1H-NMR: 0.66 (t, 6H), 0.92 (t, 6H), 1.64 (q, 4H), 2.08 (q, 4H), 2.24 (s, 3H), 2.39 (s, 3H), 6.03 (d, 1H), 6.76 (d, 1H), 7.00-7.46 (m, 6H), 7.43 (d, 2H), 8.12 (d, 2H); MS (ESI+): 467 (M-OH)+.
example 3
Synthesis of 5-(4-{1-ethyl-1-[4-(3-hydroxy-4,4-dimethyl-pentyl)-3-methyl-phenyl]-propyl}-2-methyl-phenyl)-furan-2-carboxylic acid
[0308]
(1) Synthesis of 4-{1-ethyl-1-[4-(3-hydroxy-4,4-dimethyl-1-pentynyl)-3-methyl-phenyl]-propyl}-2-methyl-phenol (racemic mixture.)
[0309]A 2.44 M solution of n-butyllithium in hexane (42.8 mL, 104.5 mmol) was added to a solution of 4-[1-ethyl-1-(4-ethynyl-3-methyl-phenyl)-propyl]-2-methyl-phenol (Example 1-(3); 12.22 g, 41.8 mmol) in tetrahydrofuran (300 mL) at 0° C., and the mixture was stirred at the same temperature for 30 minutes. Trimethylacetaldehyde (13.79 mL, 125.4 mmol) was added at 0° C., and the mixture was stirred at the same temperature for 30 minutes. A saturated aqueous ammonium chloride solution was added to the reaction mixture, followed by extraction with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by a silica gel column (hexane / ethyl acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com