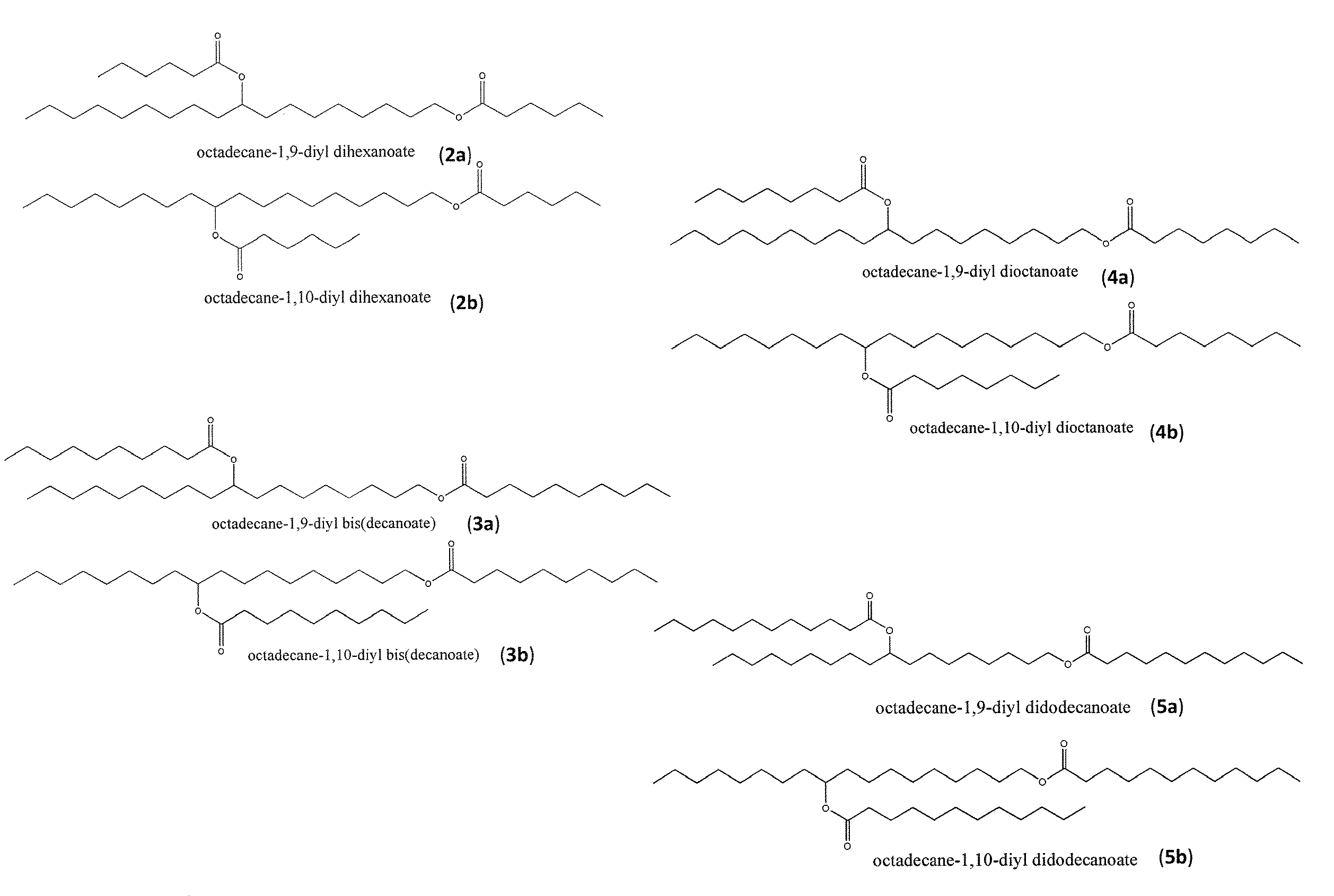
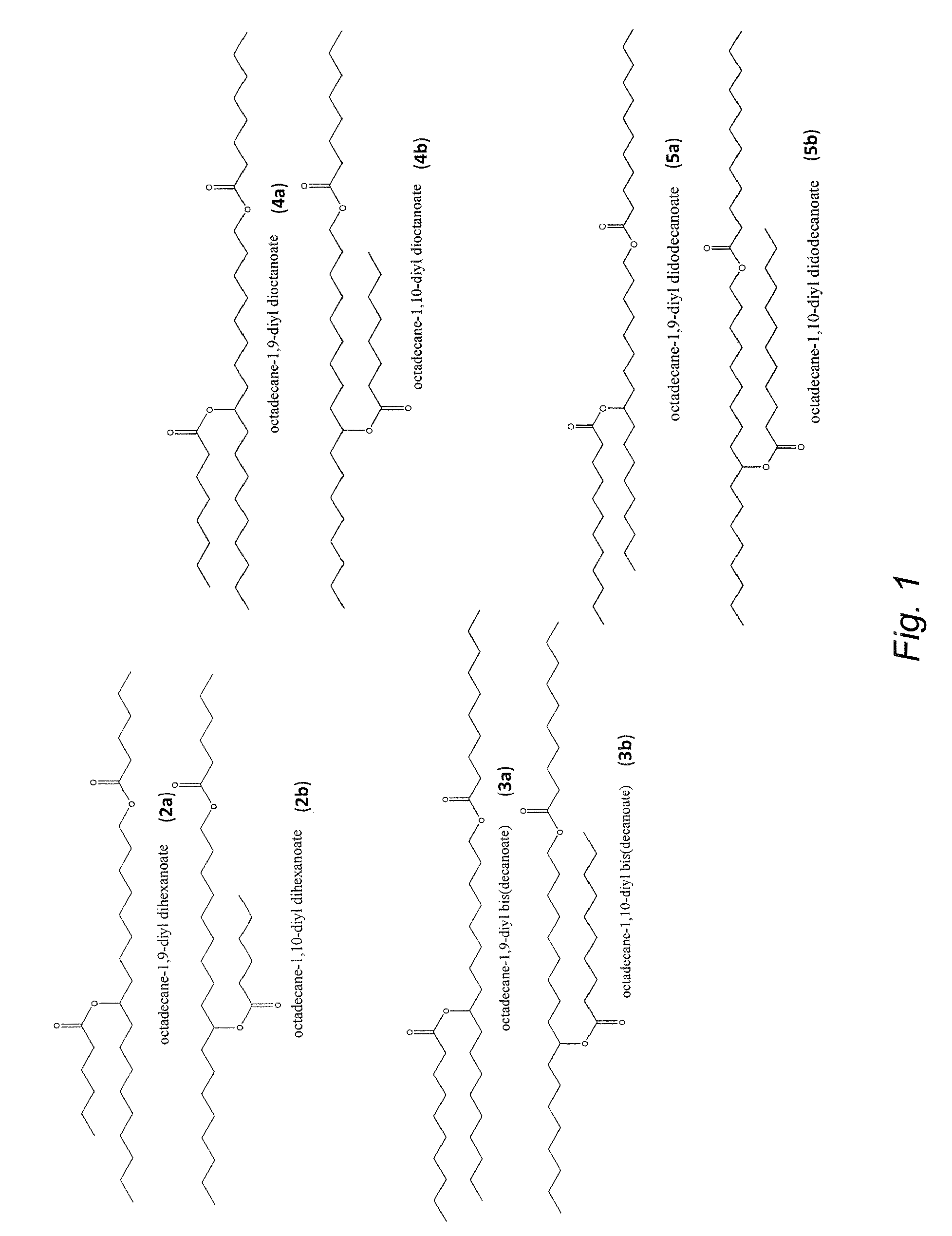
Synthesis of biolubricant esters from unsaturated fatty acid derivatives
a technology of unsaturated fatty acid and biolubricant, which is applied in the direction of fatty acid chemical modification, organic chemistry, lubricant composition, etc., can solve the problems of general compromising the oxidation stability of the lubricant composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
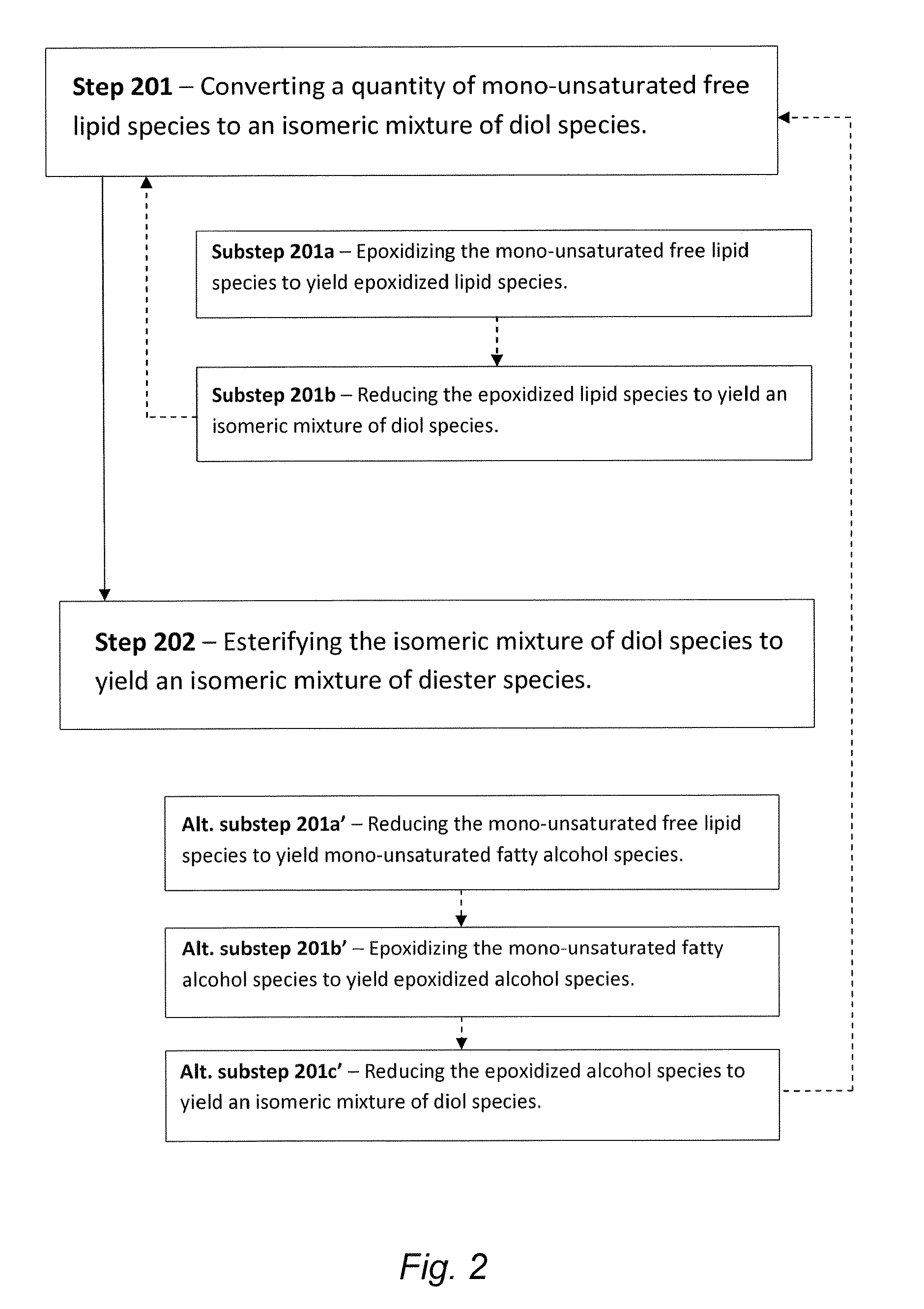
[0076]This Example serves to illustrate synthesis of mono-unsaturated fatty alcohol 11, in accordance with some embodiments of the present invention, and en route to the formation of isomeric diester mixture 4a / 4b. Referring to Scheme 3 (FIG. 5) oleic acid 7 was reduced to the corresponding oleoyl alcohol 11 (Step 501) as follows.
[0077]To an ice-cold (i.e., in an ice bath) suspension of 43 grams (1.13 mol) of lithium aluminum hydride (LiAlH4) in tetrahydrofuran (THF) in a 3-neck 3-liter reaction flask fitted with an overhead stirrer and a reflux condenser, 150 grams (0.53 mol) of oleic acid 7 was added drop-wise over a period of 45 minutes via an addition funnel. The resulting reaction mixture was allowed to warm gradually to room temperature, after which the ice bath was replaced with a heating mantle and the reaction mixture was refluxed for 4 hours. After refluxing, the reaction mixture was allowed to cool to room temperature and left to stir overnight. The reaction progress was ...
example 2
[0078]This Example serves to illustrate the synthesis of epoxy-alcohol species 12 (oleoyl epoxide), en route to the synthesis of isomeric diester mixture 4a / 4b and in accordance with some embodiments of the present invention. Referring again to Scheme 3 (FIG. 5), epoxy-alcohol species 12 was prepared from oleoyl alcohol 11 (Step 502) according to the following procedure.
[0079]To an ice-cold (ice-bath) solution of 52 grams of 75 wt % mCPBA (meta-chloro-peroxybenzoic acid) in 300 mL of methylene chloride (CH2Cl2) in a 3-neck 1L reaction flask, 50 grams of olcoyl alcohol 11, prepared as described above in Example 1, was added drop-wise over a 45 minute period. The reaction was allowed to slowly warm to room temperature, after which it was left to stir overnight at room temperature. The following day, the reaction mixture (solids+clear liquid) was filtered. The filtrate was rinsed once with 150 mL of 10 wt % NaSO3 aqueous solution, once with 200 mL of saturated KHCO3 solution, and three...
example 3
[0080]With continued reference to Scheme 3 (FIG. 5), this Example serves to illustrate how the epoxy-alcohol species 12 is converted (reduced) to an isomeric mixture of diol species 9a / 9b (Step 503), in accordance with some embodiments of the present invention. Isomeric mixture 9a / 9b was prepared according to the procedure that follows.
[0081]To an ice-cold suspension of 20 grams of LiAlH4 in 350 mL THF in 3-neck 2 L reaction flask (fitted with a reflux condenser and an overhead stirrer), 46 grams of epoxy alcohol 12 (synthesized as described above in Example 2 and dissolved in 200 mL of THF) were added drop-wise over a 30 minute period. The resulting mixture was left to warm to room temperature and then heated to reflux for few hours. The reaction was then allowed to stir at room temperature overnight. The next day, the reaction was placed in an ice-bath and diluted with 300 mL of ether. The ice-cold reaction mixture was treated by slowly adding 200 mL of 15 wt % NaOH solution follo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com