Treatment of hot flushes, vasomotor symptoms, and night sweats with sex steroid precursors in combination with selective estrogen receptor modulators
a technology of sex steroid precursors and estrogen receptors, applied in the direction of drug compositions, metabolic disorders, cardiovascular disorders, etc., can solve the problems of increasing the risk of certain diseases for female patients, discontinuing therapy, and affecting the treatment effect of females with androgenic compounds, so as to avoid the risk of endometrial cancer, prevent osteoporosis, and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
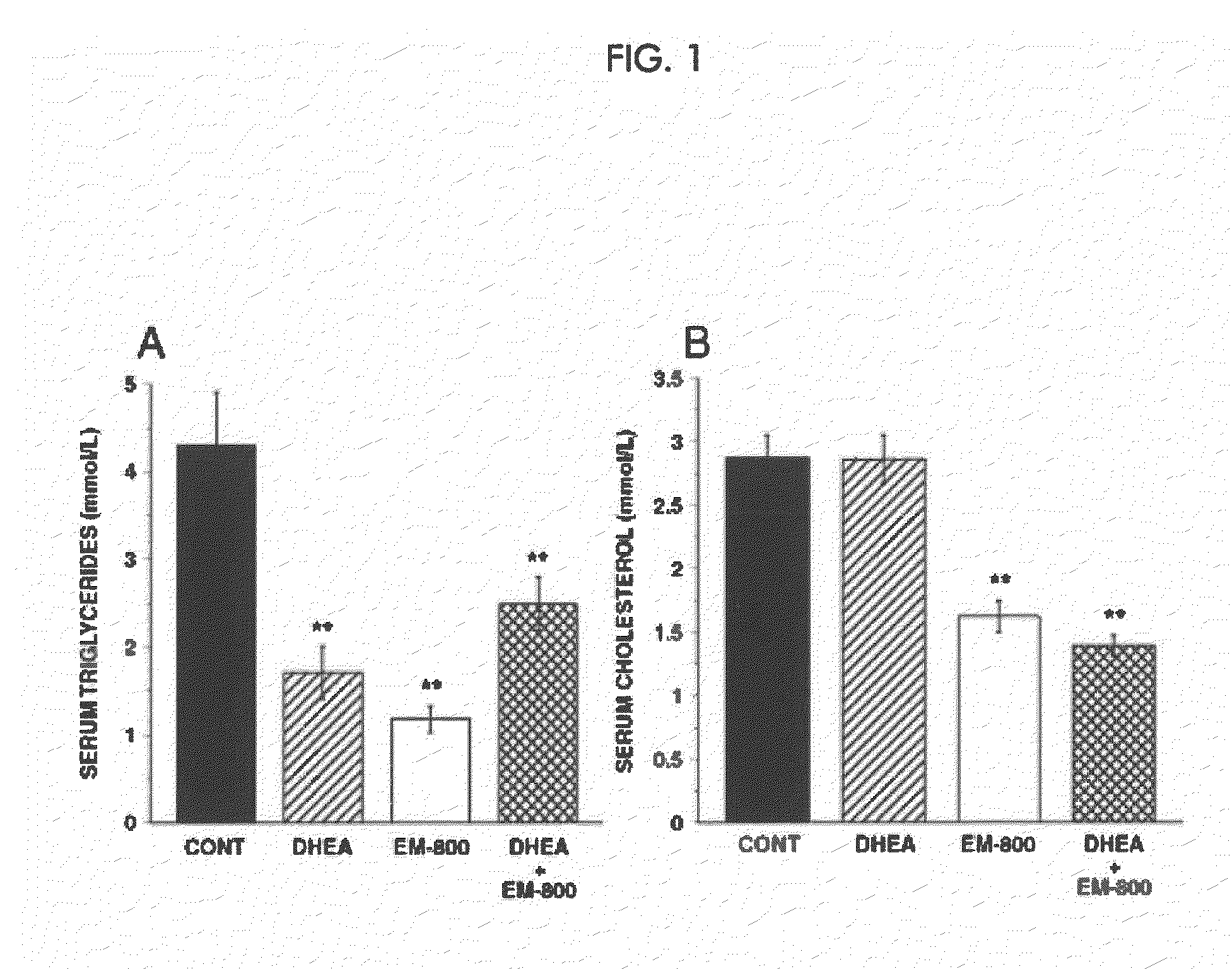
[0291]In the mammary gland, androgens are formed from the precursor steroid dehydroepiandrosterone (DHEA). Clinical evidence indicates that androgens have inhibitory effects on breast cancer. Estrogens, on the other hand, stimulate the development and growth of breast cancer. We studied the effect of DHEA alone or in combination with the newly described pure antiestrogen, EM-800, on the growth of tumor xenografts formed by the human breast cancer cell line ZR-75-1 in ovariectomized nude mice.
[0292]Mice received daily subcutaneous injections of 0.5 μg estrone (an estrogenic hormone) immediately after ovariectomy. EM-800 (15, 50 or 100 μg) was given orally once daily. DHEA was applied twice daily (total dose 0.3, 1.0 or 3.0 mg) to the dorsal skin either alone or in combination with a 15 μg daily oral dose of EM-800. Changes in tumor size in response to the treatments were assessed periodically in relation to the measurements made on the first day. At the end of the experiments, tumors...
example 2
Example of Synthesis of the Preferred Compound of the Invention
Synthesis of (S)-(+)-7-hydroxy-3-(4′-hydroxyphenyl)-4-methyl-2-(4″-(2′″-piperidinoethoxy)phenyl)-2H-1-benzopyran hydrochloride EM-01538 (EM-652, HCl)
[0308]
Step A: BF3.Et2O, toluene; 100° C.; 1 hour.
Step C: 3,4-dihydropyran, p-toluenesulfonic acid monohydrate, ethyl acetate; 25° C. under nitrogen, 16 hours, and then crystallization in isopropanol.
Steps D, E, and F:
[0309](1) piperidine, toluene, Dean & Stark apparatus, reflux under nitrogen; (2) 1,8-diazabicyclo[5,4,0]undec-7-ene, DMF, reflux 3 hours;
(3) CH3MgCl, THF, −20 to 0° C. and then room temperature for 24 hours;
Steps G, H: (1S)-(+)-10-camphorsulfonic acid, acetone, water, toluene, room temperature, 48 hours.
Step HH: 95% ethanol, 70° C., then room temperature 3 days.
Step HHR: Recycling of mother liquor and wash of step HH
(S)-10-camphorsulfonic acid, reflux; 36 hours, then room temperature for 16 hours.
Step I:
[0310](1) DMF aq., Na2CO3, ethyl acetate;
(2) Ethanol, dilu...
example 3
Materials and Methods
Animals
[0316]Female BALB / c mice (BALB / cAnNCrlBR) weighing 18-20 g were obtained from Charles-River, Inc. (St-Constant, Quebec, Canada) and housed 5 per cage in a temperature (23±1° C.)—and light (12 h light / day, lights on at 7:15)—controlled environment. The mice were fed rodent chow and tap water ad libitum. The animals were ovariectomized (OVX) under Isoflurane anesthesia via bilateral flank incisions and randomly assigned to groups of 10 animals. Ten mice were kept intact as controls.
Treatments
[0317]In the first experiment (FIGS. 11 to 14), tested compounds, namely EM-652.HCl, lasofoxifene (as free base; active and inactive enantiomers) and raloxifene, were administered orally by gavage once daily at doses of 1, 3 or 10 μg / animal for 9 days, starting 2 days after ovariectomy. In the second experiment (Table 6), TSE 424 was administered orally by gavage once daily at doses of 1, 3, 10 or 30 μg / animal for 9 days, starting 2 days after ovariectomy. In both exper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| uterine weight | aaaaa | aaaaa |
| uterine weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com