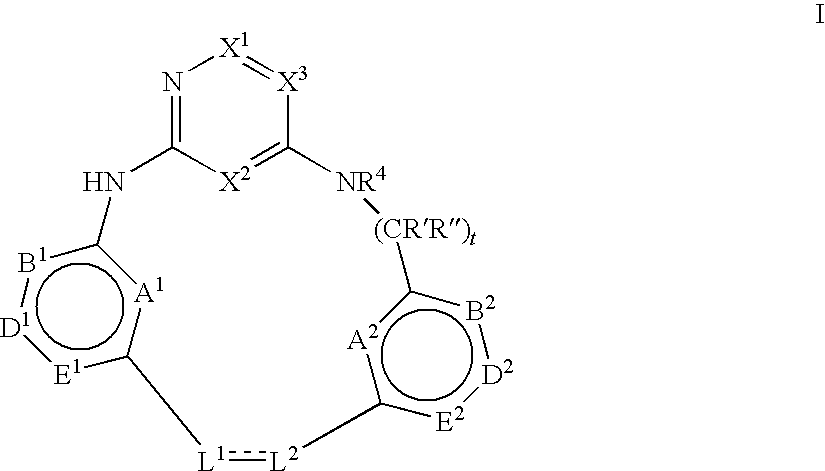
Macrocyclic compounds and their use as kinase inhibitors
a technology of macrocyclic compounds and kinase inhibitors, applied in the field of macrocyclic compounds, can solve the problems of adversely affecting patient health, poor prognosis, and elevated levels of circulating cytokines, and achieve the effect of efficient treatment and few associated clinical side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6-Chloro-15-oxa-2,4,8,23-tetraazatetracyclo[15.3.1.1(3,7).1(10,14)]tricosa-1(21),3(23),4,6,10(22),11,13,17,19-nonaene trifluoroacetate
[0674]
Step A: 2,5-Dichloro-N-(3-methoxybenzyl)pyrimidin-4-amine
[0675]
[0676]To a solution of 3-methoxybenzylamine (0.75 g, 5.4 mmol) and 2,4,5-trichloropyrimidine (1.1 g, 6.0 mmol) in N,N-dimethylformamide (20 mL) was added potassium carbonate (2.3 g, 16 mmol). The resulting mixture was stirred overnight at room temperature. The reaction was quenched with water. Ethyl acetate (“EtOAc”) was added and the layers were separated. The aqueous layer was extracted with EtOAc once. The combined organic layers were washed with water and brine successively, then dried (Na2SO4), filtered, and concentrated. The crude product was purified by silica gel column chromatography to give the desired product as a white powder (1.43 g, 92%). LCMS for C12H12ClN3O (M+H)+: m / z=284.0, 286.0.
Step B: [3-({5-Chloro-4-[(3-methoxybenzyl)amino]pyrimidin-2-yl}amino)phenyl]methanol
[06...
example 2
6-Chloro-15-oxa-2,4,8,24-tetraazatetracyclo[16.3.1.1(3,7).1(10,14)]tetracosa-1(22),3(24),4,6,10(23),11,13,18,20-nonaene trifluoroacetate
[0682]
Step A: 2-(3-Aminophenyl)ethanol
[0683]
[0684]Into a pressure bottle were added 2-(3-nitrophenyl)ethanol (3.0 g, 18 mmol) and methanol (100 mL) and 10% palladium on carbon (0.1 g, 0.08 mmol). The reaction mixture was hydrogenated at 45 psi overnight. The resultant mixture was filtered and concentrated to provide the desired product (2.44 g, 99%) as a white solid. LCMS for C8H12NO (M+H)+: m / z=138.1.
Step B: 2-[3-({5-Chloro-4-[(3-methoxybenzyl)amino]pyrimidin-2-yl}amino)phenyl]ethanol
[0685]
[0686]Into a reaction flask were added 2,5-dichloro-N-(3-methoxybenzyl)pyrimidin-4-amine (0.33 g, 1.2 mmol) (prepared according to Example 1, step A), 1,4-dioxane (20 mL), 2-(3-aminophenyl)ethanol (0.22 g, 1.6 mmol), and p-toluenesulfonic acid monohydrate (0.080 g, 0.42 mmol). The mixture was heated at 105° C. for 3 hours and then an aqueous solution of NaHCO3 (s...
example 3
6-Chloro-16-thia-2,4,8,15,23-pentaazatetracyclo[15.3.1.1(3,7).1(10,14)]tricosa-1(21),3(23),4,6,10(22),11,13,17,19-nonaene 16,16-dioxide trifluoroacetate
[0690]
Step A: tert-Butyl(3-{[(2,5-dichloropyrimidin-4-yl)amino]methyl}phenyl)carbamate
[0691]
[0692]To a solution of tert-butyl [3-(aminomethyl)phenyl]carbamate (0.50 g, 2.2 mmol) and 2,4,5-trichloropyrimidine (0.45 g, 2.5 mmol) in N,N-dimethylformamide (6 mL) was added potassium carbonate (0.62 g, 4.5 mmol). The resultant mixture was stirred overnight at room temperature. The reaction was quenched with water. EtOAc was added and the layers were separated. The aqueous layer was extracted with EtOAc twice. The combined organic layers were washed with water and brine successively, then dried (Na2SO4), filtered, and concentrated. The resulted residue was triturated with methylene chloride and hexanes to give the desired product as an off-white powder (0.75 g, 90%). LCMS for C12H11Cl2N4O2 (M-tBu+H)+: m / z=313.0, 315.0.
Step B: N-(3-Aminobenz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com