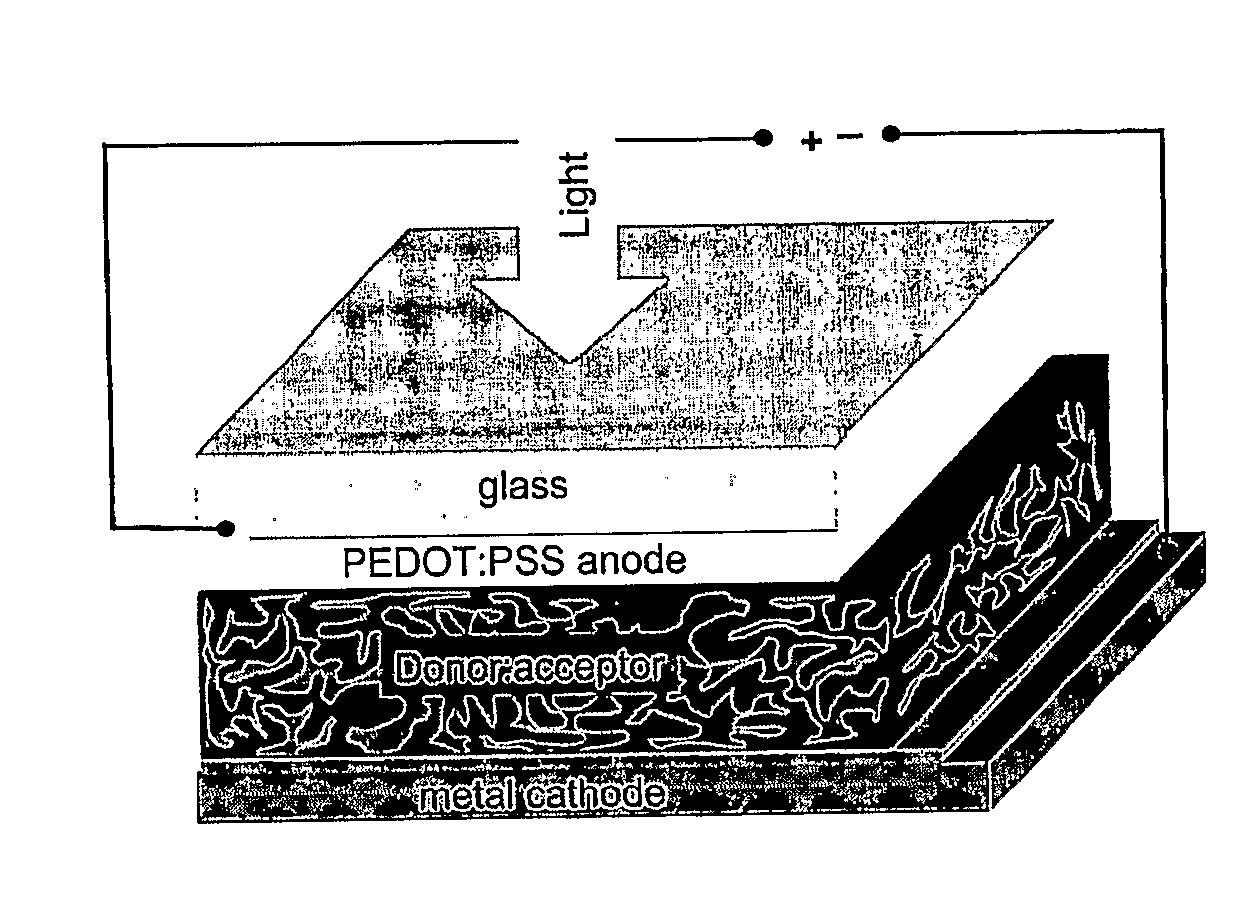
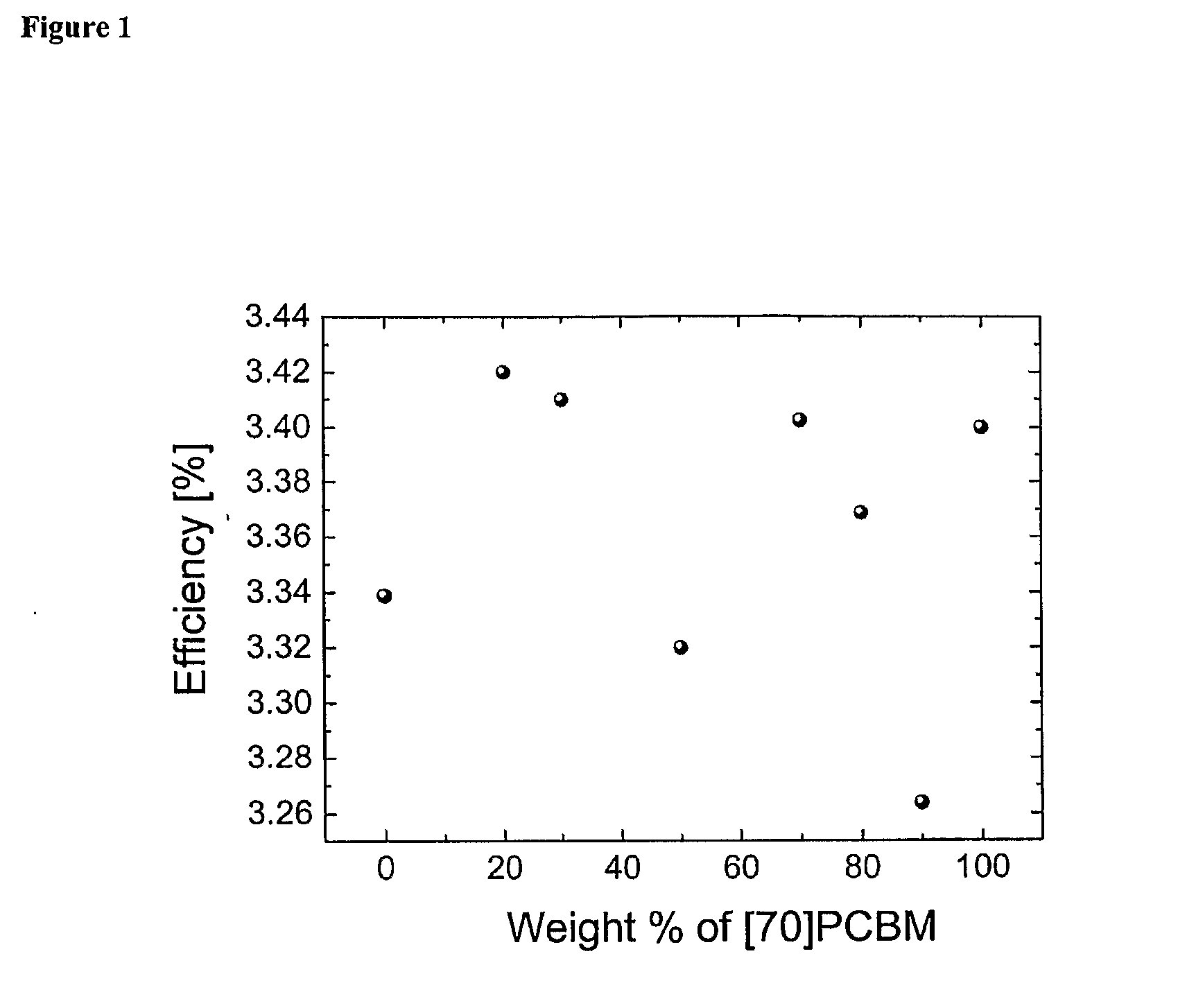
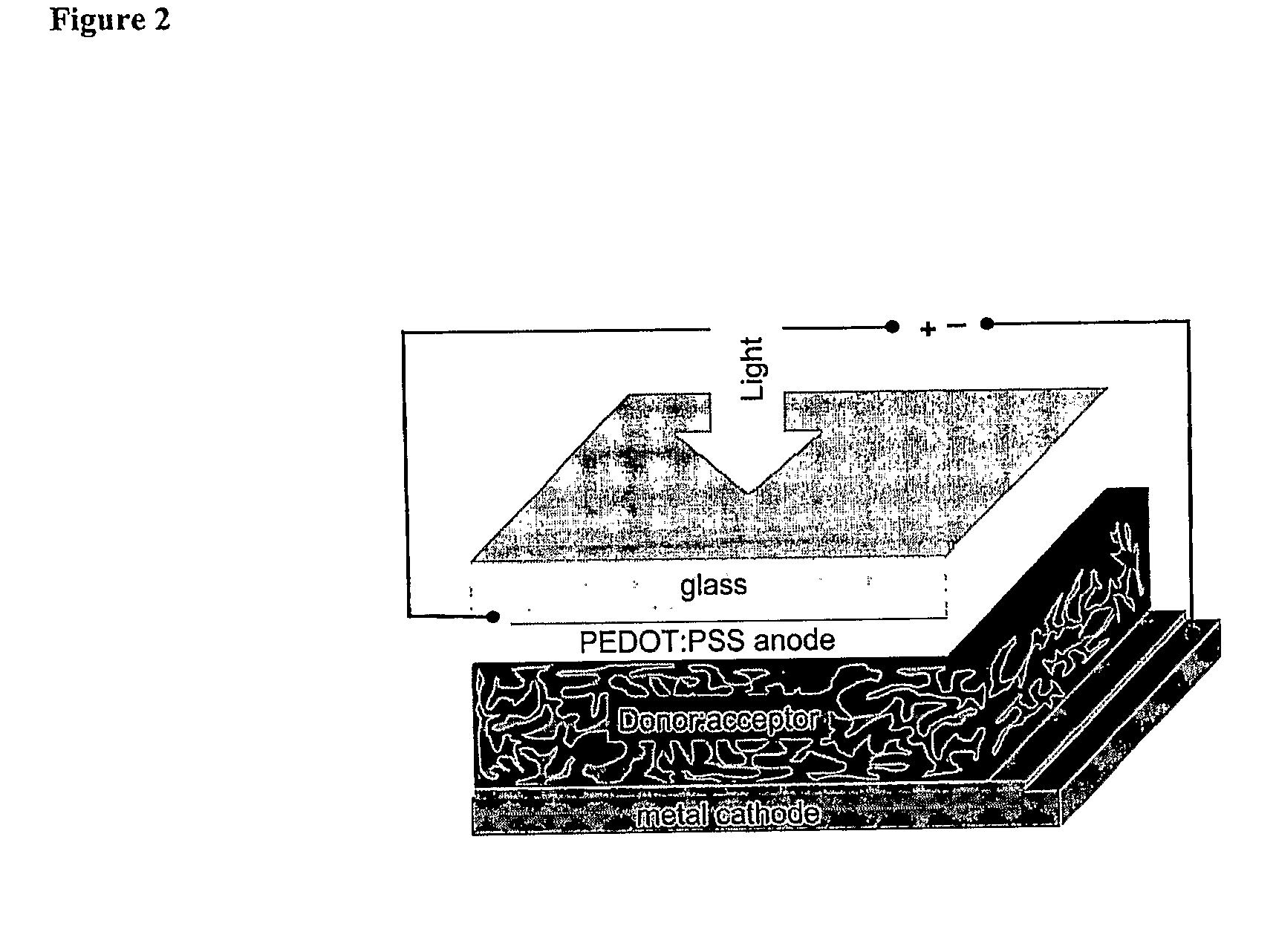
Blends of Fullerene Derivatives, and Uses Thereof in Electronic Devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
process embodiments
Additional Process Embodiments
[0571]In certain embodiments, the present invention relates to the method of synthesizing a mixture of fullerene derivatives or a pure component fullerene derivative by substituting a mixture of fullerenes for a pure fullerene in a fullerene synthesis; varying the ratio of fullerene derivative content by using a purification technique.
[0572]In certain embodiments, the present invention relates to the aforementioned method where the purification method is filtration or elution over activated charcoal.
[0573]In certain embodiments, the present invention relates to the aforementioned method where the purification method is adsorption on activated charcoal followed by filtration.
[0574]In certain embodiments, the present invention relates to the aforementioned method where the purification comprises multiple repeated steps.
[0575]In certain embodiments, the present invention relates to the aforementioned method where the purification method is preparatory scal...
example 1
Synthesis Information
Synthesis of Mixtures PCBM Derivatives ([Cn]PCBM)
[0626]Synthesis of PCBM derivatives was performed according to methods described in Hummelen et al. J. Org. Chem. 1995, 60, 532. Analysis by HPLC indicated about 74% [60]PCBM, 22% [70]PCBM, and 4% [>70]PCBM. Reduction of [>70] adducts may be performed by analytical or prep scale HPLC, using a Cosmocil Buckyprep column, or activated carbon filtration.
[0627]In a typical methanofullerene synthesis, using arc-produced fullerenes, compositions of the product before any purification are approximately 40% to 50% unreacted fullerenes, 40% to 50% [60] and [70] mono-adducts (˜9% bis, ˜1% tris), ≦1% other impurities (oxides of C60 and the derivatives; dimers of C60 and derivatives), 4% higher fullerene (>C70) derivatives and unreacted fullerenes >C70. Unreacted fullerenes may be removed with a silica gel column. Higher fullerene derivatives may be removed by analytical or prep scale HPLC, using a Cosmocil Buckyprep column, o...
example 2
Processing of Derivatized Fullerene Mixtures
Method 1:
[0637]Crude PCBM mixture, resulting from the synthesis using mixed fullerenes, was dissolved in p-xylene (10 g / L). The crude PCBM mixture typically consists of 3-6% higher PCBM adducts ([>70]PCBM), 20-27% [70]PCBM and 68-77% [60]PCBM, plus small amounts of unreacted fullerenes and trace amounts of fullerene oxides, fullerene derivative oxides, and dimers of unreacted fullerenes and fullerene derivatives. Compositions may vary depending on fullerene production method, combustion produced fullerenes typically have higher percentages of C70 and higher fullerenes.
[0638]The mixed PCBMs are purified over a bed containing 2.5 grams activated charcoal (Merck charcoal activated GR for analysis, product number: 102186) per gram PCBM, mixed with a supporting medium of Silica gel (Aldrich, Merck grade 9385, product number: 227196) in a 1:3.5 ratio (w / w), to allow for a better flow through the bed. The bed is prepared by mixing the activated c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com