Methods and systems for deacidizing gaseous mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
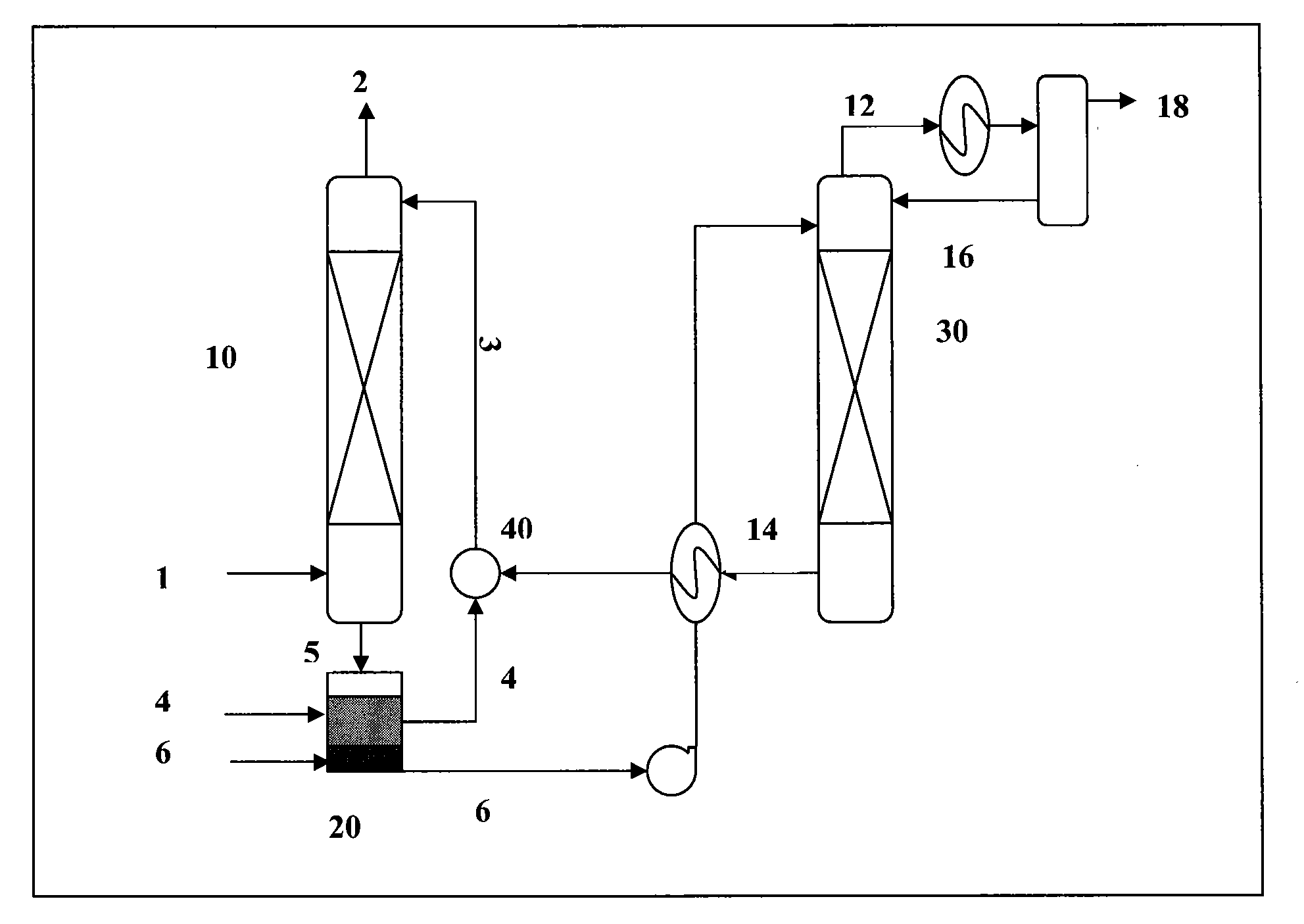
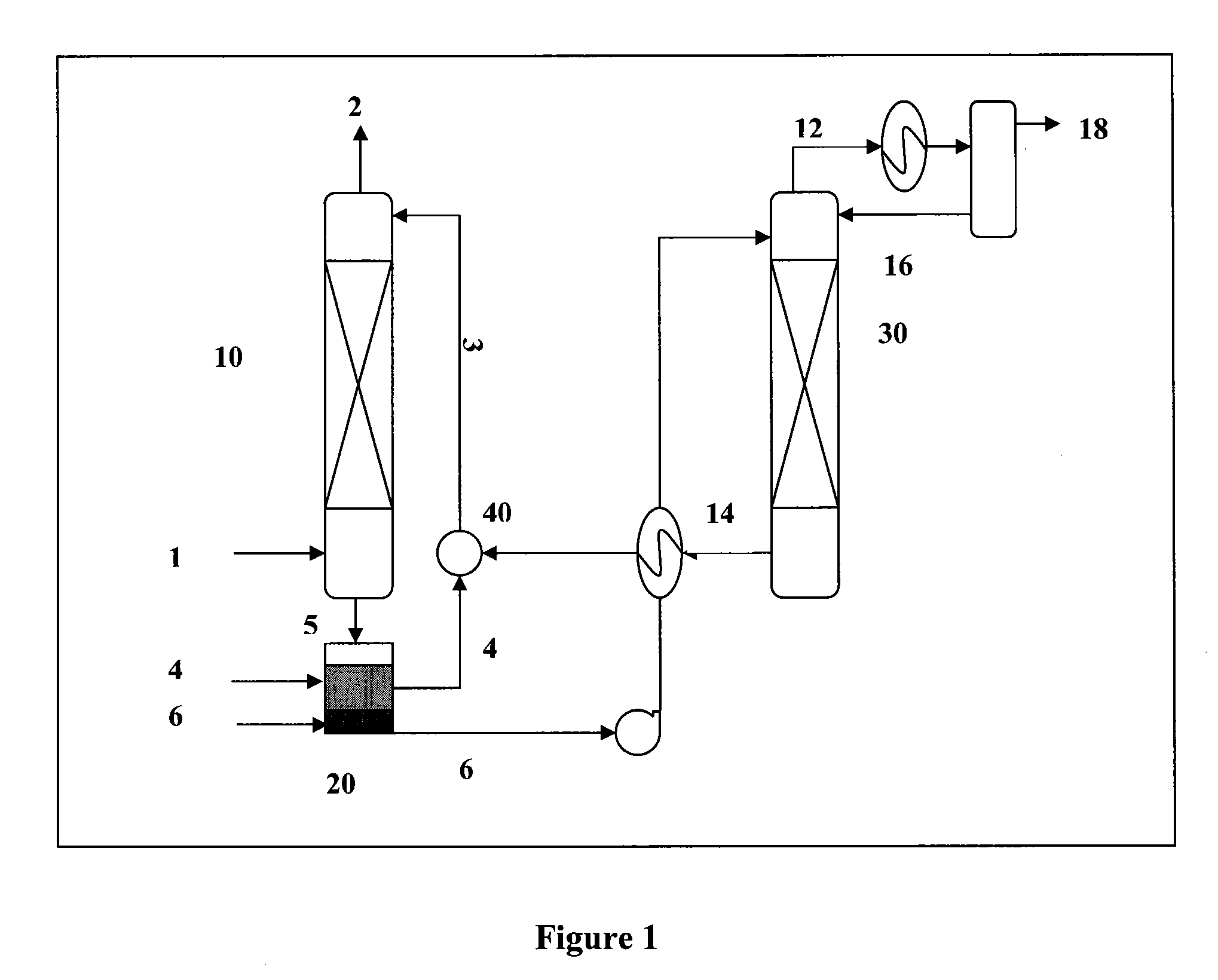
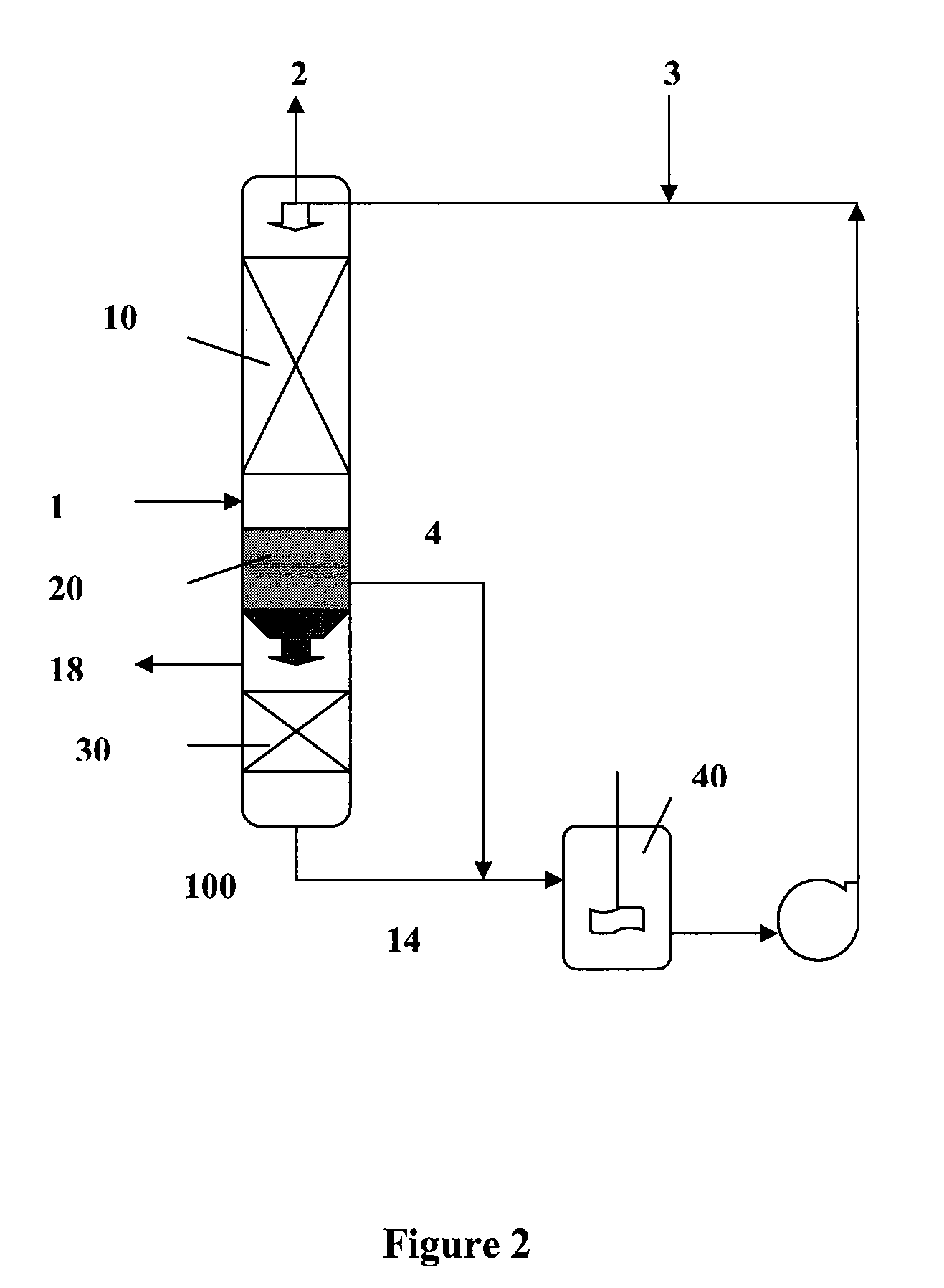
[0075]An absorbent comprising two phases, an organic phase and a carrier phase, and a gaseous mixture containing carbon dioxide were supplied into a stirring cell absorption unit at about 25-45° C., 1 atm. The organic phase comprised 20% by volume of monoethanolamine and 80% by volume of decyl alcohol. The carrier phase comprised the reaction product of monoethanolamine and carbon dioxide.
[0076]When the organic phase contacted the gaseous mixture, carbon dioxide gas was chemically absorbed into the organic phase by reacting with monoethanolamine in the organic phase. The absorbed carbon dioxide existed as a reaction product formed between the absorbed carbon dioxide and monoethanolamine. The reaction product was then transferred through the interface between the organic phase and carrier phase into the carrier phase and accumulated there.
[0077]After absorption of the carbon dioxide gas, the absorbent was settled and separated by gravity into a first gas-lean phase and a gas-rich pha...
example 2
[0080]An absorbent comprising two liquid phases, an organic phase and a carrier phase, and a gaseous mixture containing carbon dioxide were supplied into a stirring cell absorption unit at about 25-45° C., 1 atm. The organic phase comprised 20% by volume of dibutylamine and 80% by volume of isooctanol. The carrier phase comprised an aqueous solution of 150 g / l of potassium carbonate.
[0081]When the organic phase contacted the gaseous mixture, carbon dioxide gas was chemically absorbed into the organic phase by reacting with dibutylamine in the organic phase. The absorbed carbon dioxide existed as a reaction product formed between the absorbed carbon dioxide and dibutylamine. The reaction product was then transferred through the interface between the organic phase and carrier phase into the carrier phase by further reacting with potassium carbonate in the carrier phase to form potassium bicarbonate. Thus, the absorbed carbon dioxide ultimately existed as potassium bicarbonate in the c...
example 3
[0085]An absorbent comprising two liquid phases, an organic phase and a carrier phase, and a gaseous mixture containing carbon dioxide were supplied into a stirring cell absorption unit at about 25 to 45° C., 1 atm. The organic phase comprised 20% by volume of dibutylamine and 80% by volume of isooctanol. The carrier phase was a water solution.
[0086]When the organic phase contacted the gaseous mixture, carbon dioxide gas was chemically absorbed into the organic phase by reacting with dibutylamine in the organic phase. The absorbed carbon dioxide existed as a reaction product formed between the absorbed carbon dioxide and dibutylamine. The reaction product was then transferred through the interface between the organic phase and carrier phase into the carrier phase and accumulated there.
[0087]After absorption of the carbon dioxide gas, the absorbent was settled and separated by gravity into a first gas-lean phase and a gas-rich phase. The first gas-lean phase, comprising the unreacted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gravity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com