Apparatus for spectrometric based oximetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
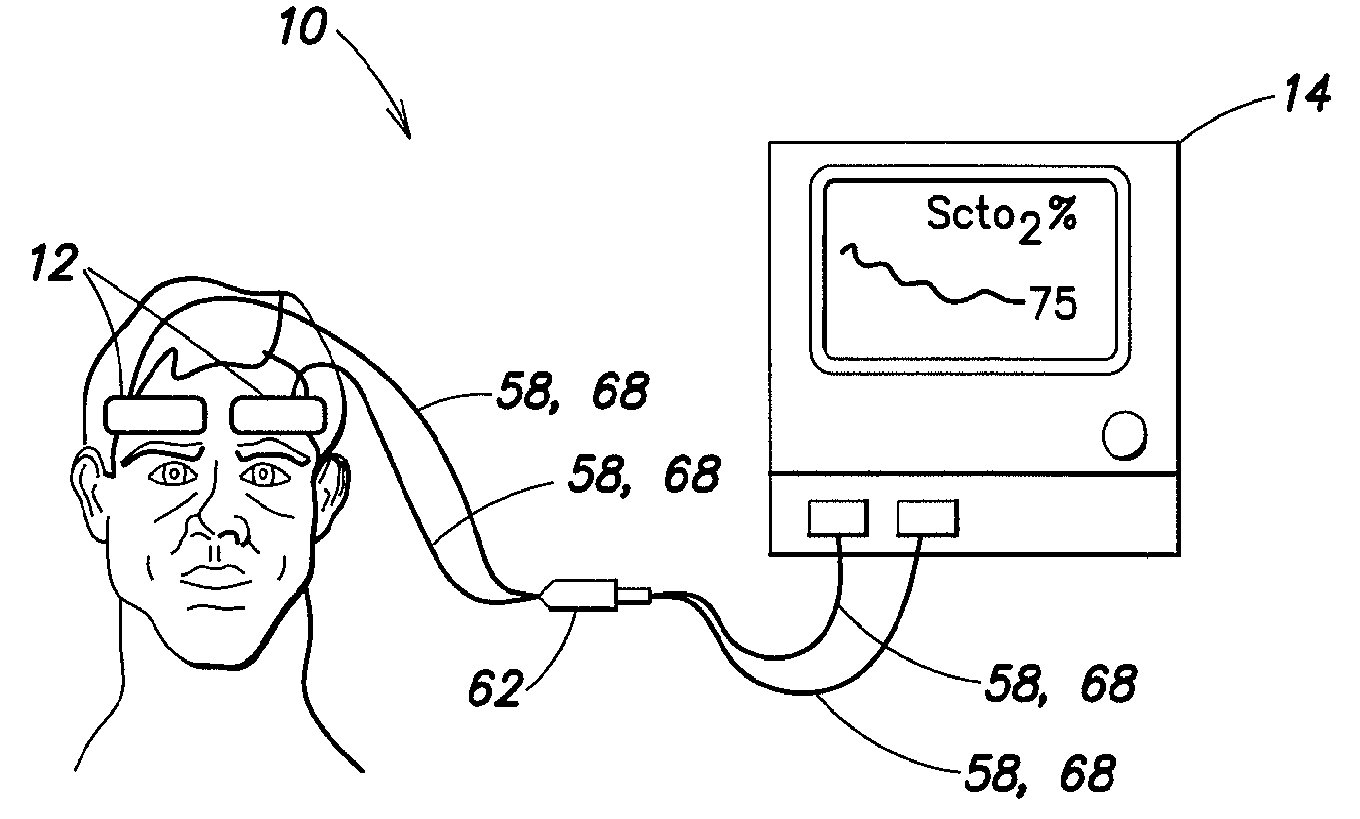
[0026]Referring now to the drawings, a near infra-red spectroscopy (NIRS) system 10 includes one or more NIRS sensor assemblies 12 connected to a NIRS system 10. The NIRS system 10 includes a processor 14 for providing signals to and / or receiving signals from the NIRS sensor assembly (ies) 12. For purposes of providing a detailed description of the present NIRS sensor assembly 12, the sensor assembly 12 will be described herein as being used in connection with the NIRS system 10 described in U.S. Pat. No. 6,456,862 and U.S. Pat. No. 7,071,701, which are examples of acceptable NIRS systems. The NIRS sensor assembly 12 is not, however, limited to use with any particular NIRS system.
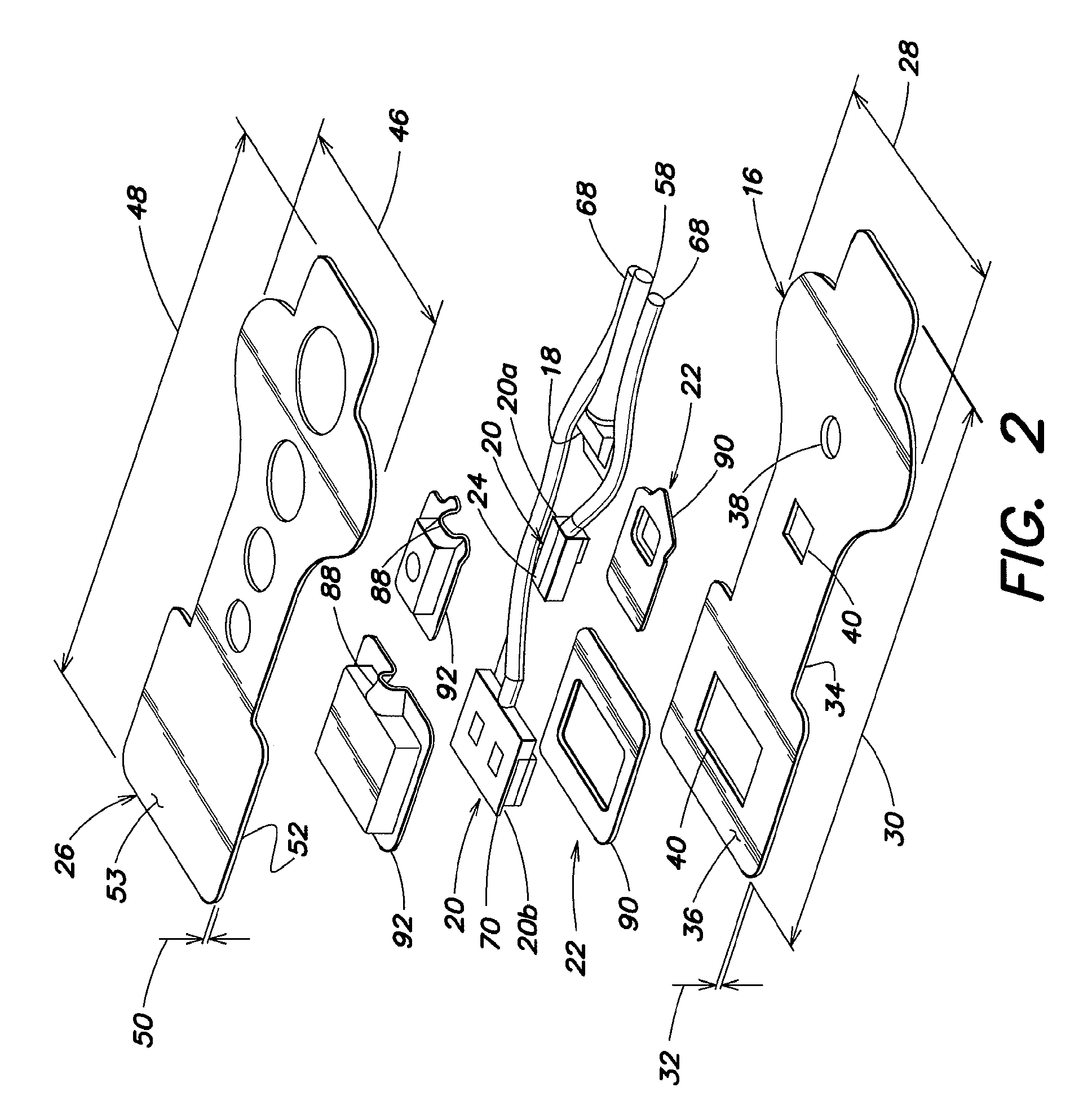
[0027]An embodiment of a NIRS sensor assembly 12 is shown in FIGS. 2-8. The NIRS sensor assembly 12 includes a pad 16, at least one light source 18, at least one light detector 20, at least one detector housing 22, electromagnetic interference (EMI) shielding 24, and a cover 26. In those embodiments of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com