Method for producing high-purity nickel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
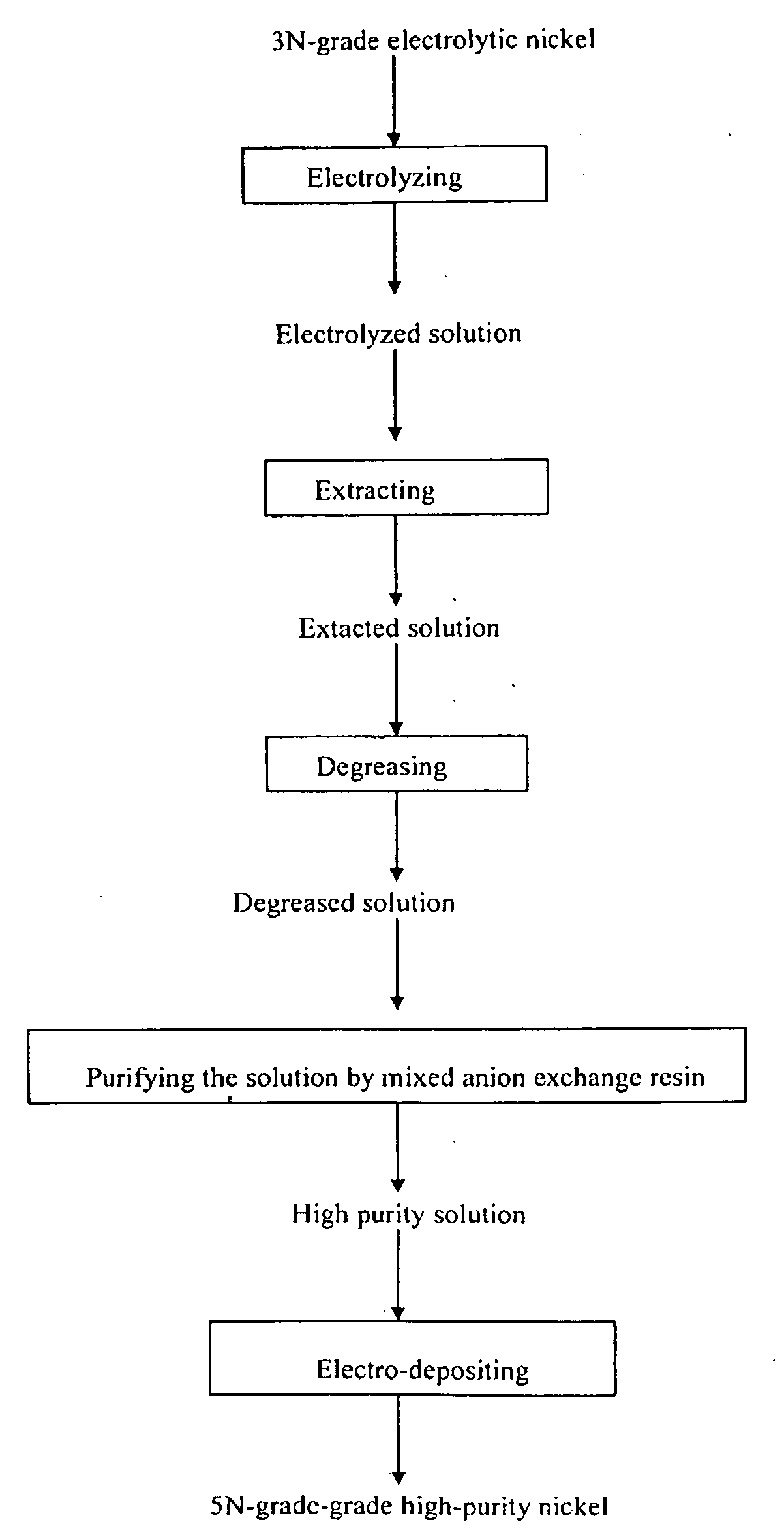
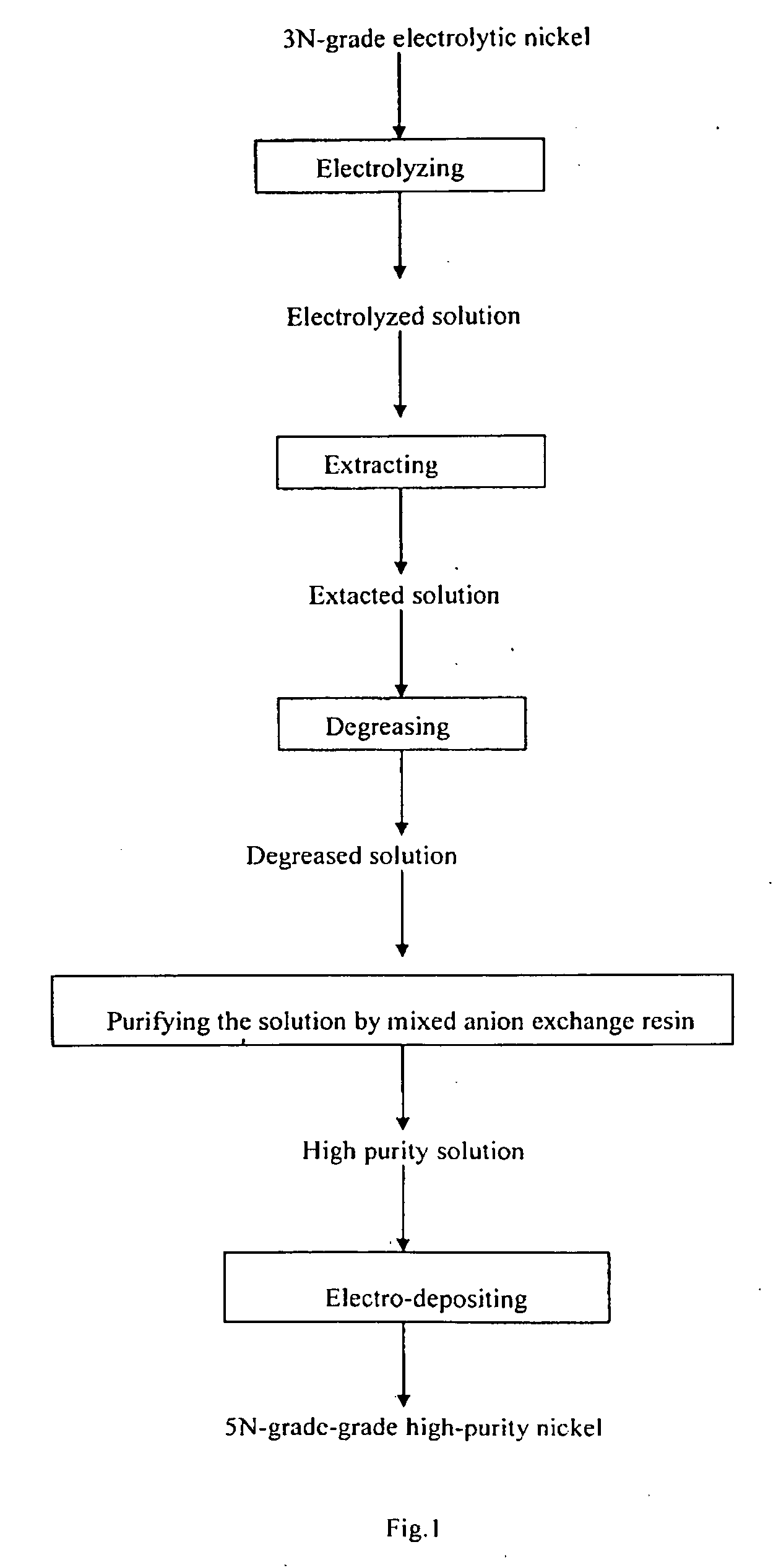
[0028]3N-grade electrolytic nickel was electrolyzed in hydrochloric acid system to prepare NiCl2 solution at a current density of 100 A / m2. When the concentration of H+ was 1 g / l, the current density was changed to 30 A / m2 and electrolyzed till the pH of the solution to 3, wherein the concentration of Cl− was 6 mol / L and the impurities were shown in the table 1.
TABLE 1Contents of impurities in original solutionunit: g / lNo.NameCoCuFePbZn1original solution0.0090.0020.0020.0010.001
[0029]Anion extractant contained 25 vol. % tertiary amine, 45 vol. % butylate, and 30 vol. % sulphonated kerosene was washed by high purity water, saturated with 4 mol / l high purity hydrochloric acid. The electrolytic solution was extracted by 3-stage countercurrent extraction at the extractant phase ratio of 1:2, and then back-extracted with pure water 10 minutes after achieving extraction equilibrium. The concentration of Co was decreased from 0.009 g / l to 0.001 g / l. The ingredients of extracting residue we...
example 2
[0032]3N-grade electrolytic nickel was electrolyzed in hydrochloric acid system to prepare NiCl2 solution at a current density of 150 A / m2. When the concentration of H+ was 1.5 g / l, the current density was changed to 50 A / m2 and electrolyzed till the pH of the solution to 3, wherein the concentration of Cl− was 6 mol / L and the impurities were shown in the table 5.
TABLE 5Contents of impurities in original solutionunit: g / lNo.ItemCoCuFePbZn1original solution0.0080.0030.0010.0010.001
[0033]Anion extractant contained 40 vol. % tertiary amine, 20 vol. % butylate, and 40 vol. % sulphonated kerosene was washed by high purity water, saturated with 4 mol / l high purity hydrochloric acid. The electrolytic solution was extracted by 3-stage countercurrent extraction at the extractant phase ratio of 1:2, and then back-extracted with pure water 10 minutes after achieving extraction equilibrium. The concentration of Co was decreased from 0.008 g / l to 0.001 g / l. The ingredients of extract residue wer...
example 3
[0036]Employed electrolytic solution such as example 2 and the anion extractant contained 20 vol. % tertiary amine, 45 vol. % butylate, and 35 vol. % sulphonated kerosene, Ingredients in solution further purified by ion change were shown in table 8.
TABLE 8Contents of impurities in solution purified by ion exchangeunit: g / lNo.ItemCoCuFePbZn1Solution purified0.00010.0001by ion change
[0037]An insoluble anode was employed to electro-deposit in NiCI2 solution. Electro-deposition conditions were as follows: current density was 200 Am2, pH of NiCl2solution was 2. Electro-deposition temperature was 60°. An insoluble anode was employed to electro-deposit the solution further purified by ion exchange to produce 5N-grade high purity nickel. Main impurities of the produced high purity nickel were as follows: alkali metals less than 0.1 ppm, Fe, Co and Cr less than 1 ppm each, U and Th less than 0.1 ppb each, C less than 60 ppm, and O less than 100 ppm. Contents of main impurities in high purity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com