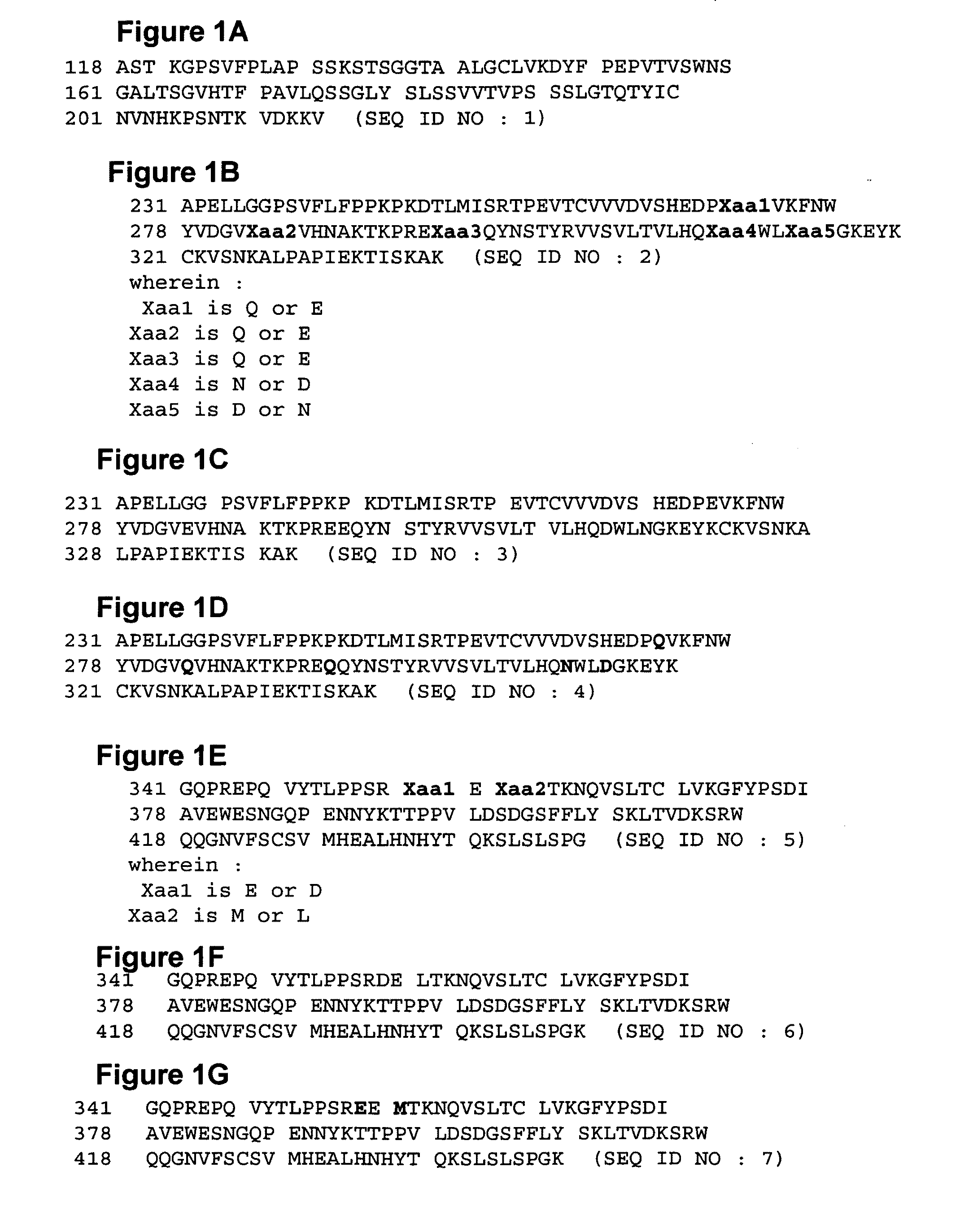
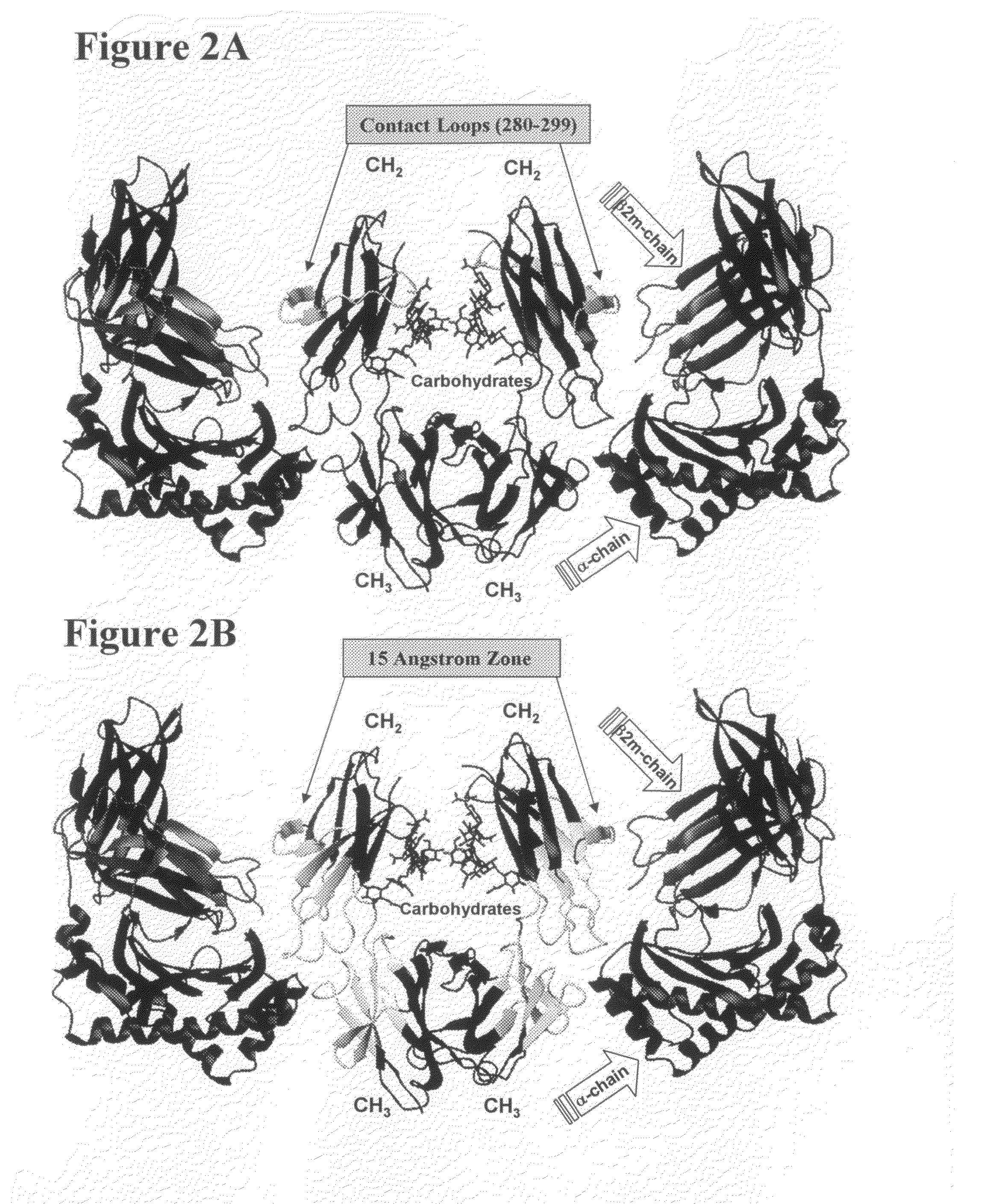
Altered polypeptides, immunoconjugates thereof, and methods related thereto
a technology of immunoconjugates and polypeptides, applied in the field of altered polypeptides, immunoconjugates thereof, and methods related thereto, can solve the problems of reducing the functionality, specificity, affinity or avidity of polypeptides, and the chemistry of cross-linking reactions is usually very inefficient,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Altered Polypeptides Comprising Engineered Cysteines
a) Design of Engineered Cysteine Residues in the CH3 Domain of Human IgG1:
[0492]The three dimensional structure of an human IgG1 Fc region was examined and amino acid positions or “sites” where surface-accessible cysteine residues could be engineered in the CH3 domain were evaluated by the following criteria: (1) the amino acid side-chain of the CH3 domain is exposed and solvent accessible; (2) the site is not required for binding of antigen, FcγR, FcRn, Protein A or Protein G; and (3) there is a low risk of disulfide formation with the counterpart cysteine residue in the other heavy chain of the symmetric Fc domain.
[0493]From a candidate list of R355, T359, K360, N361, N384, Q386, N389, D413, S415, Q418, V422, K439, S440, S442, L443, S444, and K 446b (EU numbering as in Kabat) a final list of 5 amino acid positions was chosen based on (1) compatibility of a cysteine residue with the backbone and sidechain configuration a...
example 2
Production of Altered Antibodies Comprising Engineered Cysteines
a) Production of an Altered Antibody Comprising Engineered Cysteine Residues in the CH3 Domain of Human IgG1:
[0495]Using a yeast expression vector for a His6-Fc (human IgG1) protein, site directed mutagenesis with synthetic oligonucleotides was performed using the QuikChange mutagenesis kit of Stratagene (the 6×His tag is disclosed as SEQ ID NO: 59). The plasmid pKS528 was used a DNA template for mutagenesis (FIG. 9). pKS528 contains the Fc region of human IgG1 fused to the alpha factor secretion signal of Saccharomyces cerevisiae, with a His6 tag (SEQ ID NO: 59) at the N-terminus of the Fc region. This plasmid is an expression vector for Pichia pastoris. pK3528 also comprises a synthetic promoter having seven TetO repeats and the minimal AOX1 promoter of Pichia pastoris. The promoter can be induced with doxycyline in a P. pastoris strain expressing the reverse Tet transactivator (rTA). The P. pastoris strain MMC216 exp...
example 3
Production of altered Fab fragments comprising Engineered Cysteines
[0501]Site-directed mutagenesis CH1 and CL domains was carried out in using the E. coli expression plasmid XW335 as a template (FIG. 17). XW335 encodes both Heavy and Light chain regions for humanized CBE11 (anti-lymphotoxin beta receptor) and is engineered for expression of Fabs in E. coli. The araBAD promoter drives expression of the Heavy and Light chains of the Fab and the ompA signal sequence is present at the N-terminus of both the Heavy and Light chains and provides for secretion into the periplasm of E. coli. The amino acid (SEQ ID NO: 24) and nucleotide (SEQ ID NO: 25) sequences of the heavy chain of CBE11 Fab are depicted in FIG. 18. The amino acid (SEQ ID NO: 26) and nucleotide (SEQ ID NO: 27) sequences of the light chain of CBE11 Fab are depicted in FIG. 19. Mutagenesis by the QuikChange method (with oligonucleotides as shown in Table 3) was carried out and candidates were confirmed by DNA sequence analys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com