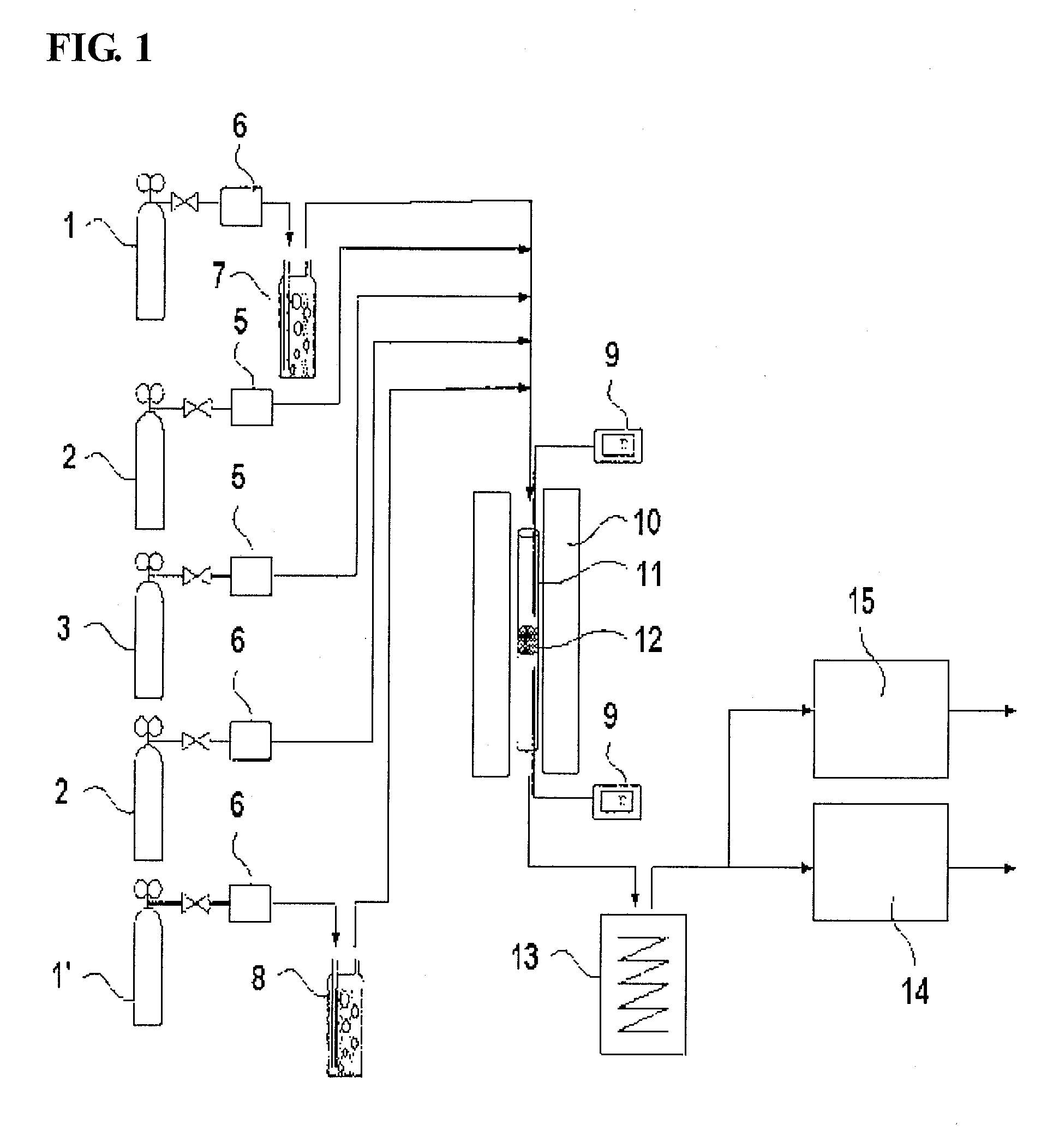
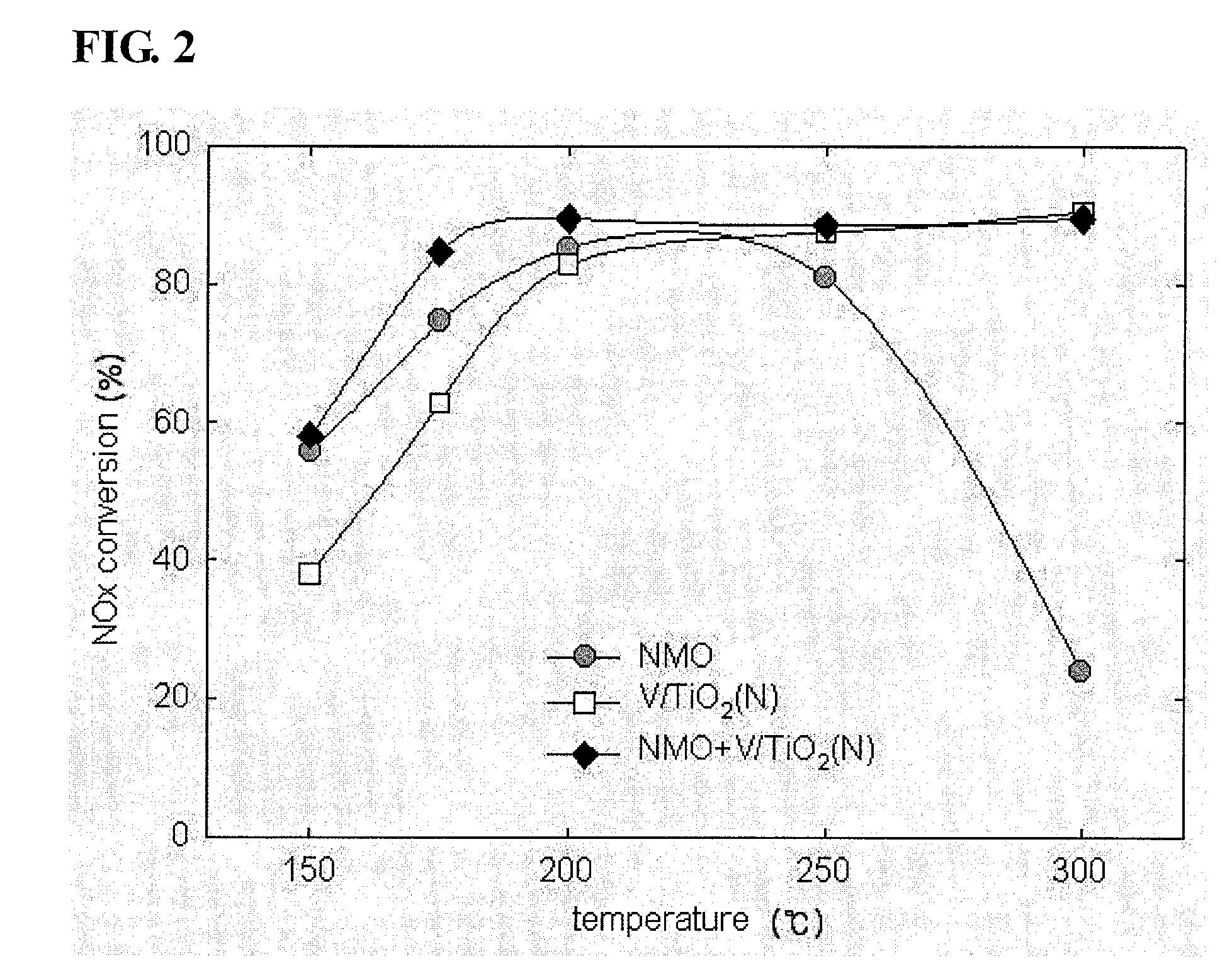
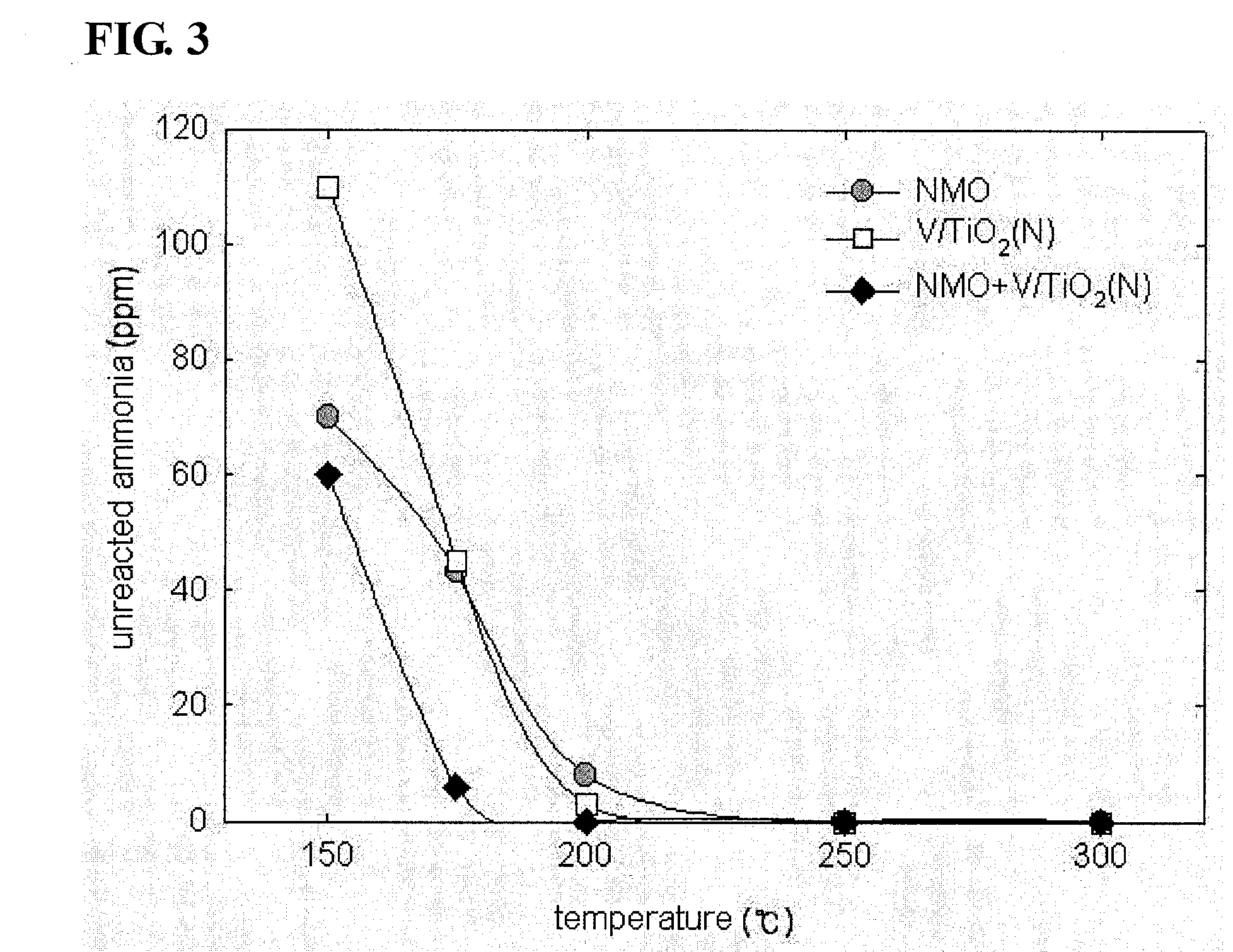
Vanadium/Titania Catalyst Comprising Natural Manganese Ore for Removing Nitrogen Oxides and Dioxin in Wide Operating Temperature Range and Method of Using the Same
a technology of natural manganese ore and catalyst, which is applied in the direction of physical/chemical process catalyst, metal/metal-oxide/metal-hydroxide catalyst, etc., can solve the problems of reducing visibility distance, affecting the activity of catalyst, and affecting the health of human body, so as to achieve excellent nitrogen oxide removal, excellent denitrification performance, and remove dioxin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 3
Preparation of V / TiO2 (W) with Tungsten
[0072]12.35 g of ammonium tungstate ((NH4)2WO4) was dissolved in 30 ml of distilled water, and the resulting solution was heated to about 60° C. to completely dissolve it. After the solution was cooled to room temperature, 100 g of titania was added thereto to obtain a slurry. The slurry was heated to about 70° C. while stirring it to evaporate water therefrom. After the completion of the evaporation of water, a drying process was conducted at about 120° C. for 24 hours and then a calcination process was conducted at 500° C. for 10 hours in an air atmosphere, thus preparing a tungsten-titania mixed support. Subsequently, the same procedure as in Preparative Example 2 was conducted, resulting in a V / TiO2 catalyst having tungsten supported thereon, which is referred to as V / TiO2 (W).
example 1
[0073]The NMO of Preparative Example 1 was mixed with the V / TiO2 (N) catalyst of Preparative Example 2 through a ball milling process, thus preparing a mixed catalyst. The V / TiO2 (N) catalyst and NMO were mixed at a weight ratio of 10:1 and then ball milled.
example 2
[0074]A mixed catalyst was prepared in the same manner as in Example 1, with the exception that the NMO of Preparative Example 1 and the V / TiO2 (W) catalyst of Preparative Example 3 were used.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com