Metal alkoxides, apparatus for manufacturing metal alkoxides, related methods and uses thereof
a technology for metal alkoxides and metal alkoxides, applied in the direction of group 3/13 element organic compounds, group 5/15 element organic compounds, separation processes, etc., can solve the problem of limiting the extent of reaction by equilibria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
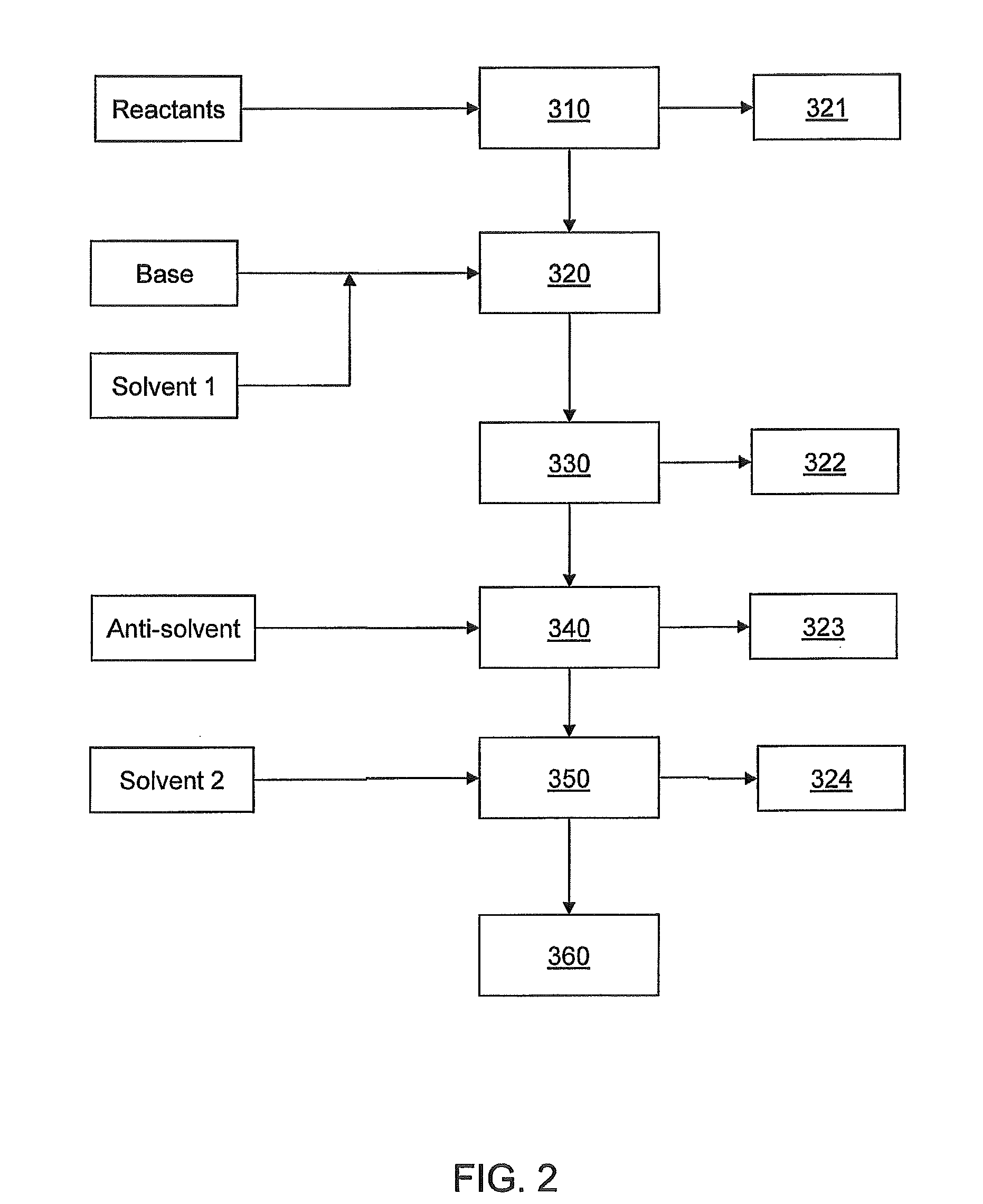
[0103]A solution of titanium oxychloride (TiOCl2, 400 grams (g)), glycerol (400 g) and water (distilled, 400 g) was fed continuously by a metering pump into the reaction distillation zone at a rate of 1.75 liters / minute (L / min). Heating (at about 80° C.) under reduced pressure at about 98.2 kPa (about 29″ Hg) gave rise to a vapor phase containing essentially water and about 20 to about 30% hydrochloric acid that was continually withdrawn and collected by vapor condensation. The viscous liquid phase product was continuously withdrawn from the reactor and treated to remove residual acid and excess glycerol. The resulting free flowing white powder product weighed about 340 g. Analysis of the product was consistent with titanyl glycerolates as confirmed by elemental analysis, 1H- and 13C-NMR.
example 2
[0104]A solution of hydrated zinc acetate (Zn(CH3CO2)2*4H2O, 400 g), glycerol (400 g), and water (distilled, 400 g) is fed continuously by a metering pump into the reaction distillation zone at a rate of 1.75 L / min. Heating under reduced pressure evolves initially water then acetic acid vapors that are continually withdrawn and collected by vapor condensation. The viscous liquid phase product is continuously withdrawn from the reactor and treated to remove residual acid and excess glycerol. The resulting free flowing white powder product when analyzed will be consistent with zinc(II) glycerolate.
example 3
[0105]A solution of hydrated manganese acetate (Mn(CH3CO2)2*2H2O, 400 g), glycerol (400 g), and water (distilled, 400 g) is fed continuously by a metering pump into the reaction distillation zone at a rate of 1.75 L / min. Heating under reduced pressure evolves initially water then acetic acid vapors that are continually withdrawn and collected by vapor condensation. The viscous liquid phase product is continuously withdrawn from the reactor and treated to remove residual acid and excess glycerol. The resulting pink powder product when analyzed will be consistent with manganese(II) glycerolate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com