Formation of self-assembled monolayers on silicon substrates
a silicon substrate and monolayer technology, applied in the field of semiconductor fabrication, can solve the problems of degrading organic molecules and producing fairly toxic materials, and achieve the effect of preventing charge leakage and suitability mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
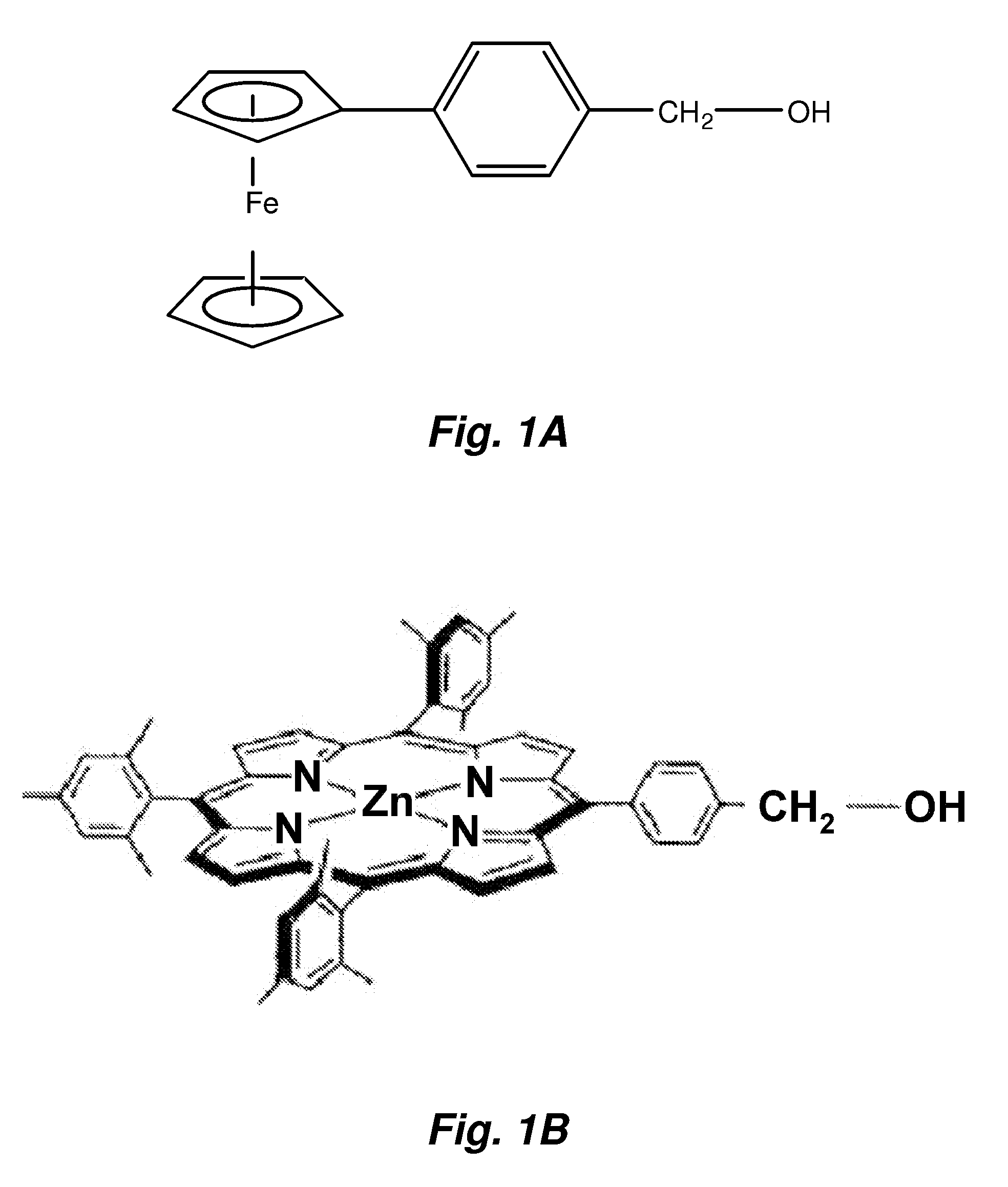
[0173]This example illustrates an approach to form SAMs of alcohol-terminated ferrocenes and porphyrins on Group IV element (e.g. Si) surfaces. In this instance, the molecules are attached to the Si surface via the formation of a Si—O bond. The new procedure has been successfully tested on both p and n-doped Si surfaces. The assemblies were stable under ambient conditions and could be exposed to repeated electrochemical oxidation and reduction cycling.
Experimental
[0174]A) Synthesis of Alcohol-Terminated Ferrocenes and Porphyrins.
[0175]Alcohol-terminated ferrocenes and porphyrins were synthesized as described in U.S. Pat. Nos. 6,272,038, 6,212,093, and 6,208,553, and in PCT Publication WO 01 / 03126. The alcohol-terminated ferrocene and porphyrin illustrated in FIGS. 1A and 1B, respectively were used in the experiments described herein.
[0176]B) Formation of SAMs.
[0177]FIG. 2 shows a schematic of one preferred embodiment of the assembly process. A Si(100) wafer (either n or p-doped) was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com