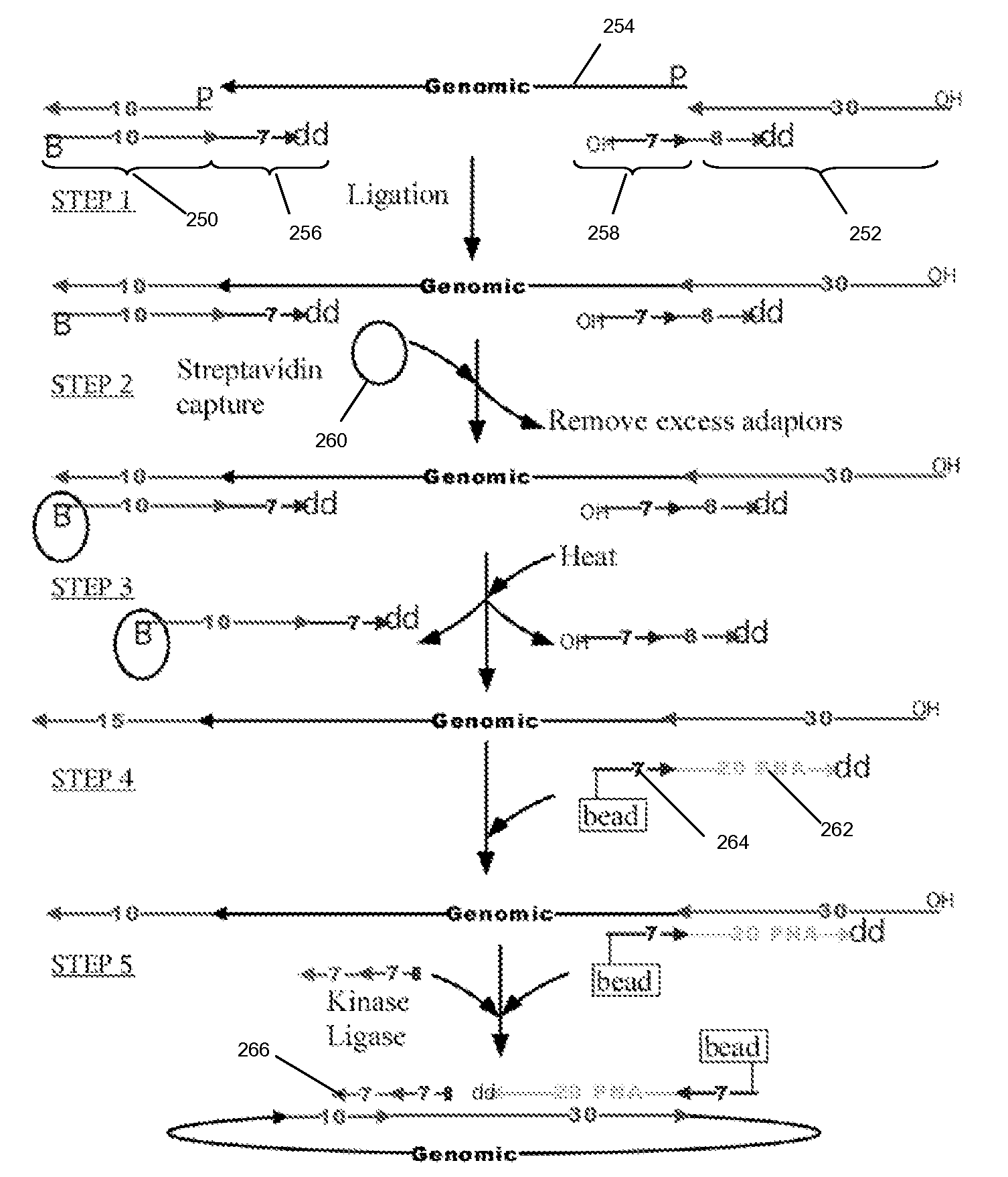
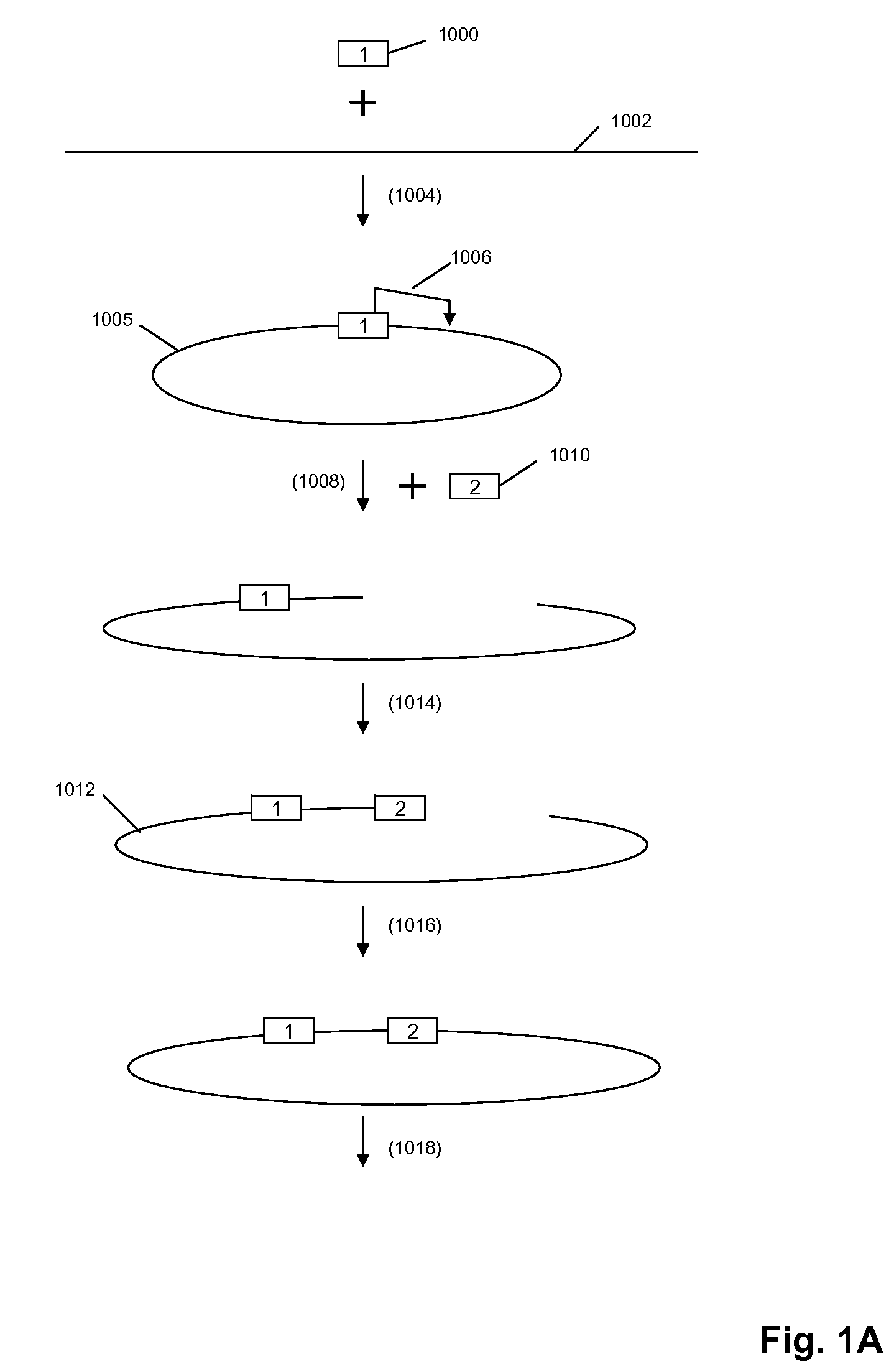
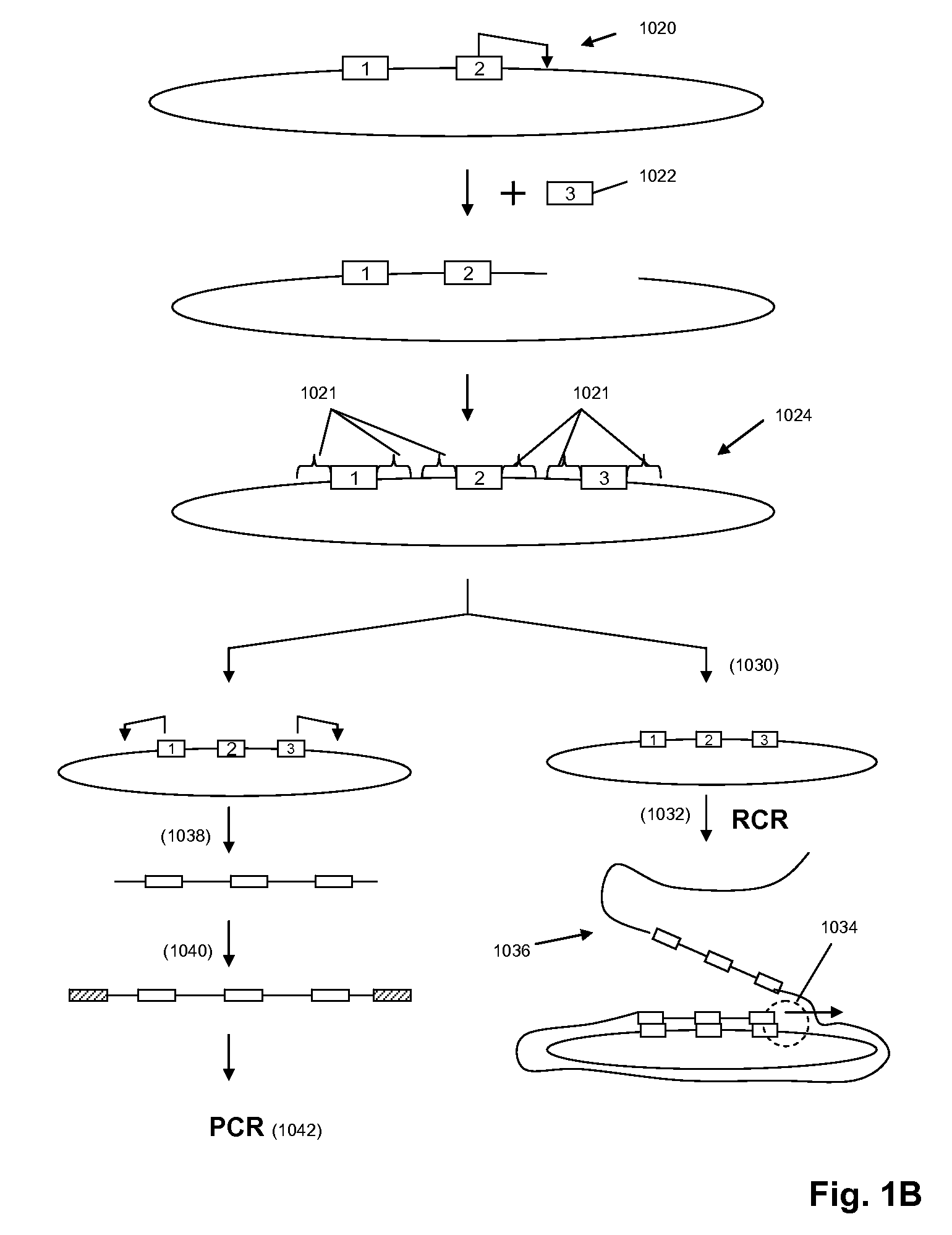
High throughput genome sequencing on DNA arrays
a genome sequencing and high throughput technology, applied in the field of high throughput genome sequencing on dna arrays, can solve the problems of reducing the packing efficiency of target targets and placing a limit on overall sequencing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
RCR Based Formation and Attachment of DNBs
[0305]Two synthetic targets were co-amplified. About one million molecules were captured on the glass surface, and then probed for one of the targets. After imaging and photo-bleaching the first probe, the second target was probed. Successive hybridization with amplicon specific probes showed that each spot on the array corresponded uniquely to either one of the two amplicon sequences. It was also confirmed that the probe could be removed through heating to 70° C. and then re-hybridized to produce equally strong signals.
example 2
Validation of Circle Formation and Amplification
[0306]The circle formation and amplification process was validated using E. coli DNA (FIG. 6). A universal adaptor, which also served as the binding site for capture probes and RCR primer, was ligated to the 5′ end of the target molecule using a universal template DNA containing degenerate bases for binding to all genomic sequences. The 3′ end of the target molecule was modified by addition of a poly-dA tail using terminal transferase. The modified target was then circularized using a bridging template complementary to the adaptor and to the oligo-dA tail.
example 3
Validation of Ligation with Condensed Concatemers
[0307]The ability for probe ligation to occur with the condensed concatemers was tested. Reactions were carried out at 20° C. for 10 min using ligase, followed by a brief wash of the chamber to remove excess probes. The ligation of a 6-mer and a labeled 5-mer produced signal levels comparable to that of an 1-mer. Software modules, including image analysis of random arrays, were tested on simulated data for whole genome sequence reconstruction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com