Optical coupler for in vivo examination of biological tissue
an optical coupler and biological tissue technology, applied in the direction of instruments, catheters, fluorescence/phosphorescence, etc., can solve the problems of inability to use pulse oximeters, inability to detect errors, etc., to achieve excellent coupling of ligh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
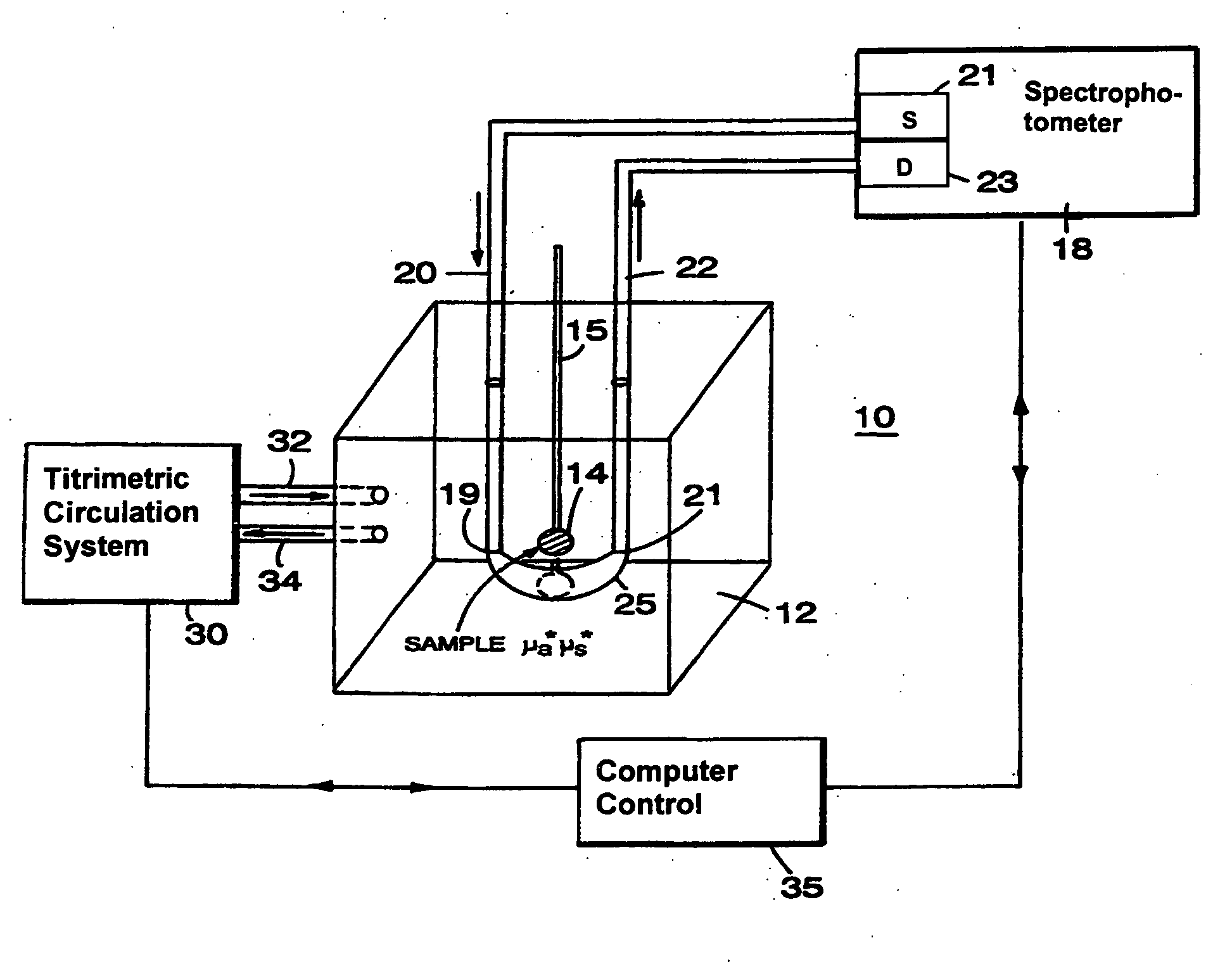
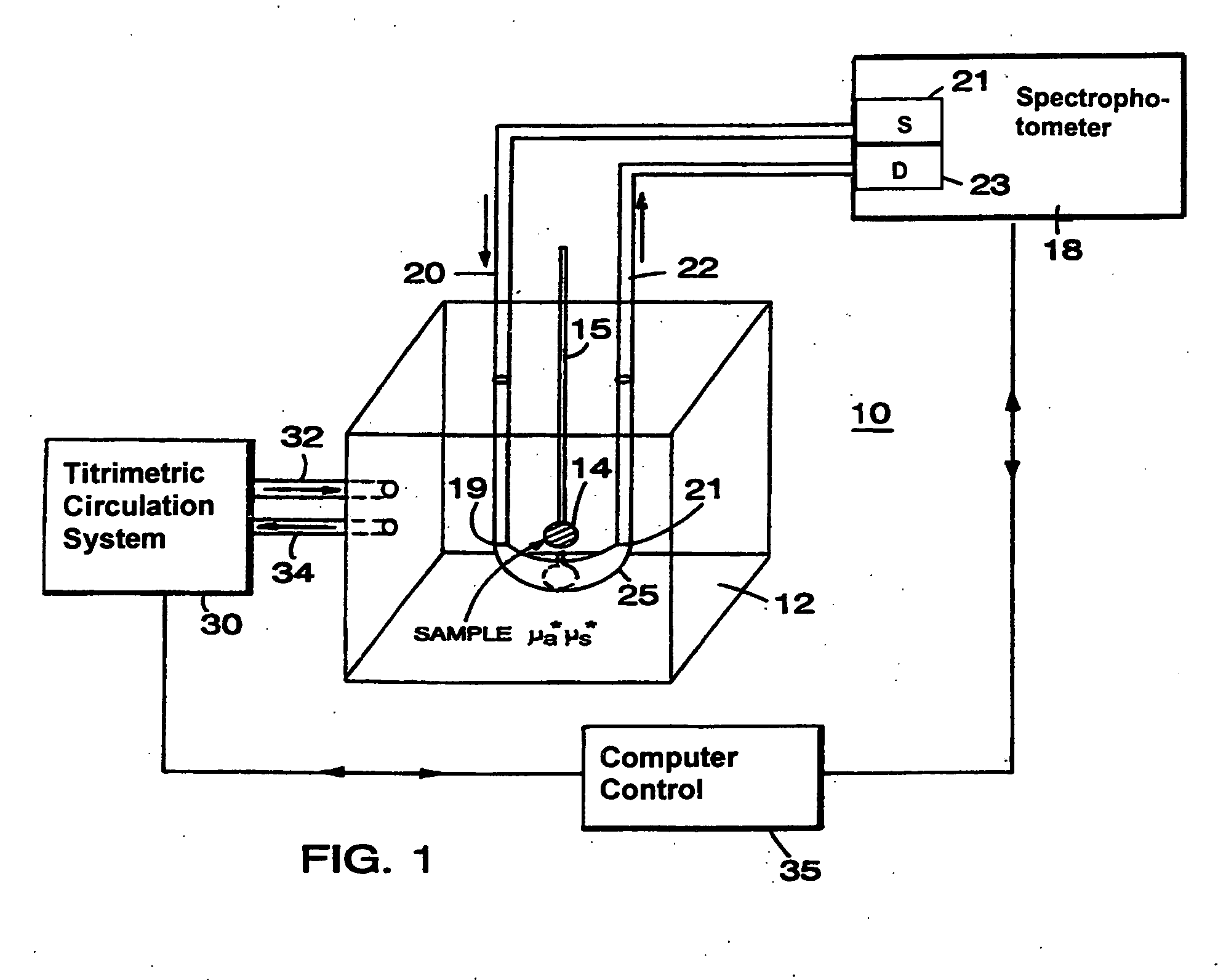
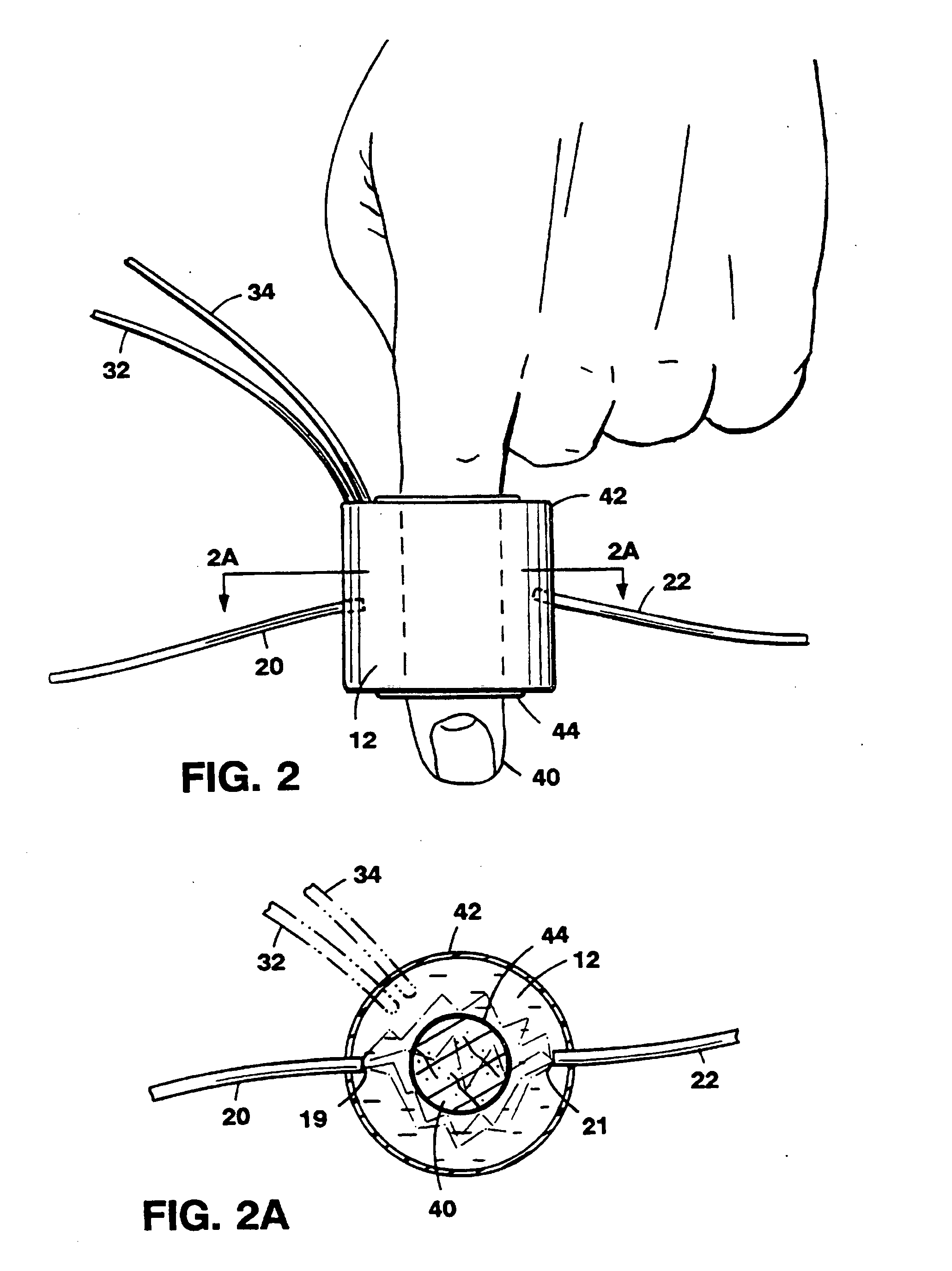
[0057]Referring to FIG. 1, the basic principle of operation of different optical couplers is explained by describing a system 10. System 10, designed for examination of biological tissue of a relatively small volume, includes an optical medium 12 of selectable optical properties, a spectrophotometer 18, a titrimetric circulation system 30, and computer control 35. Biological tissue of interest 14, attached to a locator 15, is immersed in optical medium 12. Spectrophotometer 18 examines optical properties of medium 12 by employing visible or infra-red light conducted via light guides 20 and 22. Light guides 20 and 22, which in a preferred embodiment are optical fibers, are connected to a light source 21 and a light detector 23, respectively. Photons introduced at an optical input port 19 migrate in medium 12 through a scattering and absorptive path and are detected at a detection port 21. The selectable fixed geometry of input port 19 and detection port 21 controls the migration path...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com